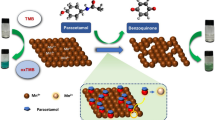
Abstract
This study describes the determination of trace levels of antimony(III) by UV–Vis spectrophotometer after preconcentration by the deep eutectic solvent/dithizone probe-based liquid–liquid microextraction method. Ditizone was used as a ligand to form the coordinated antimony complex before extraction in the preconcentration process. In the microextraction method developed in the study, deep eutectic solvent was used to dissolve the complexing agent; thus, the complexation was performed at the same time as the extraction of antimony complex by deep eutectic solvent. All variables likely to affect the ligand-antimony(III) complex, extraction efficiency, and spectroscopic measurement were optimized to lower the detection limit. Under the determined optimum conditions, the detection limit for Sb was calculated as 1.6 × 10−3 mg/L. The detection limit obtained with the method is much lower than the value obtained in the Uv–Vis spectrophotometer with the traditional method. In this study, the percent relative standard deviation for the lowest concentration was calculated as 3.12% (n = 8). This value indicates that the analysis performed has high precision. The applicability of the method was determined by performing spiked recovery tests on tap water taken from different regions. Satisfactory recovery results were obtained between 91 and 105% at three different concentrations.




Similar content being viewed by others
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Author has read, understood, and complied as applicable with the statement on “Ethical responsibilities of Authors” as found in the Instructions for authors and is aware that with minor exceptions, no changes can be made to authorship once the paper is submitted.
References
Akhtar, A., Kazi, T.G., Afridi, H.I., Baig, J.A., & Khan, M. (2020). Simultaneous preconcentration of toxic elements in eye makeup products through single drop ionic liquid based non-dispersive microextraction method using narrow glass column: Multivariate application. Microchemical Journal, 157, 104963. https://doi.org/10.1016/j.microc.2020.104963.
Akkaya, E., Chormey, D. S., & Bakırdere, S. (2017). Sensitive determination of cadmium using solidified floating organic drop microextraction-slotted quartz tube-flame atomic absorption spectroscopy. Environmental Monitoring and Assessment, 189, 513. https://doi.org/10.1007/s10661-017-6232-8
Atsever, N., Borahan, T., Bakırdere, E. G., & Bakırdere, S. (2020). Determination of iron in hair samples by slotted quartz tube-flame atomic absorption spectrometry after switchable solvent liquid phase extraction. Journal of Pharmaceutical and Biomedical Analysis, 186, 113274. https://doi.org/10.1016/j.jpba.2020.113274
Barragan, J. A., Ponce de León, C., & Alemán Castro, J. R. (2020). A. Peregrina-Lucano, F. Gómez-Zamudio, E.R. Larios-Durán, Copper and antimony recovery from electronic waste by hydrometallurgical and electrochemical techniques. ACS Omega, 5, 12355–12363. https://doi.org/10.1021/acsomega.0c01100
Biata, N. R., Mashile, G. P., Ramontja, J., Mketo, N., & Nomngongo, P. N. (2019). Application of ultrasound-assisted cloud point extraction for preconcentration of antimony, tin and thallium in food and water samples prior to ICP-OES determination. Journal of Food Composition and Analysis, 76, 14–21. https://doi.org/10.1016/J.JFCA.2018.11.004
Biata, N.R., Nyaba, L., Ramontja, J., Mketo, N., Nomngongo, P.N. (2017). Determination of antimony and tin in beverages using inductively coupled plasma-optical emission spectrometry after ultrasound-assisted ionic liquid dispersive liquid-liquid phase microextraction. Food Chemistry, 237, 904–911. https://doi.org/10.1016/j.foodchem.2017.06.058.
Bodur, S., & Bakırdere, E. G. (2019). Simultaneous determination of selected herbicides in dam lake, river and well water samples by gas chromatography mass spectrometry after vortex assisted binary solvent liquid phase microextraction. Microchemical Journal, 145, 168–172. https://doi.org/10.1016/J.MICROC.2018.10.033
Bodur, S., Erarpat, S., Dalgıç Bozyiğit, G., Selali Chormey, D., Öz, E., Özdoğan, N., & Bakırdere, S. (2020). A sensitive determination method for trace bisphenol A in bottled water and wastewater samples: Binary solvent liquid phase microextraction-quadrupole isotope dilution-gas chromatography-mass spectrometry. Microchemical Journal, 159, 105532. https://doi.org/10.1016/j.microc.2020.105532.
Borahan, T., Unutkan, T., Zaman, B. T., Bakırdere, E. G., & Bakırdere, S. (2020). Determination of copper in quince samples with a matrix matching strategy using vortex assisted deep eutectic solvent-based emulsification liquid phase microextraction–slotted quartz tube–flame atomic absorption spectrometry. Analytical Letters, 53, 2748–2760. https://doi.org/10.1080/00032719.2020.1757689
Chormey, D. S., & Bakırdere, S. (2018). Chapter Seven - Principles and recent advancements in microextraction techniques. Comprehensive Analytical Chemistry, 81, 257–294. https://doi.org/10.1016/bs.coac.2018.03.011
Duan, W., Xu, C., Liu, Q., Xu, J., Weng, Z., Zhang, X., Basnet, T.B., Dahal, M., & Gu, A. (2020). Levels of a mixture of heavy metals in blood and urine and all-cause, cardiovascular disease and cancer mortality: A population-based cohort study. Environmental Pollution, 263, 114630. https://doi.org/10.1016/j.envpol.2020.114630.
Fırat, M., Bakırdere, S., Fındıkoğlu, M. S., Kafa, E. B., Yazıcı, E., Yolcu, M., Büyükpınar, Ç., Chormey, D. S., Sel, S., & Turak, F. (2017). Determination of trace amount of cadmium using dispersive liquid-liquid microextraction-slotted quartz tube-flame atomic absorption spectrometry. Spectrochimica Acta, Part B: Atomic Spectroscopy, 129, 37–41. https://doi.org/10.1016/J.SAB.2017.01.006
Gad, S.C. (2014). Antimony. Encyclopedia of Toxicology (Third Edition), 274-276. https://doi.org/10.1016/B978-0-12-386454-3.00815-0
Gao, Y., Sturgeon, R. E., Mester, Z., Hou, X., Zheng, C., & Yang, L. (2015). Direct determination of trace antimony in natural waters by photochemical vapor generation ICPMS: Method optimization and comparison of quantitation strategies. Analytical Chemistry, 87, 7996–8004. https://doi.org/10.1021/acs.analchem.5b02001
González, M. J. G., Renedo, O. D., & Martínez, M. J. A. (2005). Simultaneous determination of antimony(III) and antimony(V) by UV-vis spectroscopy and partial least squares method (PLS). Talanta, 68(1), 67–71. https://doi.org/10.1016/j.talanta.2005.04.059
Gopalakrishnan, G., Wang, S., Mo, L., Zou, J., & Zhou, Y. (2020). Distribution determination, risk assessment, and source identification of heavy metals in mangrove wetland sediments from Qi’ao Island, South China. Regional Studies in Marine Science, 33, 100961. https://doi.org/10.1016/j.rsma.2019.100961.
Hassan, M., Erbas, Z., Alshana, U., Soylak, M. (2020). Ligandless reversed-phase switchable-hydrophilicity solvent liquid–liquid microextraction combined with flame-atomic absorption spectrometry for the determination of copper in oil samples. Microchemical Journal, 156, 104868. https://doi.org/10.1016/j.microc.2020.104868.
Li, J., Zheng, B., He, Y., Zhou, Y., Chen, X., Ruan, S., Yang, Y., Dai, C., & Tang, L. (2018). Antimony contamination, consequences and removal techniques: A review. Ecotoxicology and Environmental Safety, 156, 125–134. https://doi.org/10.1016/j.ecoenv.2018.03.024.
Mendil, D., Bardak, H., Tuzen, M., & Soylak, M. (2013). Selective speciation of inorganic antimony on tetraethylenepentamine bonded silica gel column and its determination by graphite furnace atomic absorption spectrometry. Talanta, 107, 162–166. https://doi.org/10.1016/j.talanta.2013.01.010
Mohammed, A.M. (2021). Elemental analysis using atomic absorption spectroscopy. European Journal of Engineering Research, 6, 48–51. https://doi.org/10.24018/ejeng.2021.6.7.2639
Mourya, A., Mazumdar, B., Sinha, S.K. (2019). Determination and quantification of heavy metal ion by electrochemical method. Journal of environmental chemical engineering, 7, 103459. https://doi.org/10.1016/j.jece.2019.103459.
Öztürk Er, E., Maltepe, E., Bakırdere S. (2018). A novel analytical method for the determination of cadmium in sorrel and rocket plants at ultratrace levels: Magnetic chitosan hydrogels based solid phase microextraction-slotted quartz tube-flame atomic absorption spectrophotometry. Microchemical Journal, 143, 393–399. https://doi.org/10.1016/j.microc.2018.08.019.
Panhwar, A. H., Tuzen, M., Hazer, B., & Kazi, T. G. (2018). Solid phase microextraction method using a novel polystyrene oleic acid imidazole polymer in micropipette tip of syringe system for speciation and determination of antimony in environmental and food samples. Talanta, 184, 115–121. https://doi.org/10.1016/j.talanta.2018.03.004
Pytlakowska, K., Kocot, K., Hachuła, B., Pilch, M., Wrzalik, R., & Zubko, M. (2020). Determination of heavy metal ions by energy dispersive X-ray fluorescence spectrometry using reduced graphene oxide decorated with molybdenum disulfide as solid adsorbent. Spectrochim. Acta Part B At. Atomic Spectroscopy, 167, 105846. https://doi.org/10.1016/j.sab.2020.105846.
Rahman, L., Corns, W.T., Bryce, D.W., Stockwell, P.B. (2000). Determination of mercury, selenium, bismuth, arsenic and antimony in human hair by microwave digestion atomic fluorescence spectrometry. Talanta, 52, 833–843. https://doi.org/10.1016/S0039-9140(00)00436-7.
Saleh, T. A., Sarı, A., & Tuzen, M. (2017). Effective adsorption of antimony(III) from aqueous solutions by polyamide-graphene composite as a novel adsorbent. Chemical Engineering Journal, 307, 230–238. https://doi.org/10.1016/j.cej.2016.08.070
Saleh, T. A., Tuzen, M., & Sarı, A. (2022). Effective antimony removal from wastewaters using polymer modified sepiolite: Isotherm kinetic and thermodynamic analysis. Chemical Engineering Research and Design, 184, 215–223. https://doi.org/10.1016/j.cherd.2022.05.045
Santos, V. S., de Santos, W., & J. R., Kubota, L. T., & Tarley, C. R. T. (2009). Speciation of Sb(III) and Sb(V) in meglumine antimoniate pharmaceutical formulations by PSA using carbon nanotube electrode. Journal of Pharmaceutical and Biomedical Analysis, 50(2), 151–157. https://doi.org/10.1016/j.jpba.2009.04.008
Sanz, J., Gallarta, F., Galban, J., & Castillo, J. R. (1988). Antimony determination by hydride generation - UV-visible molecular absorption spectrophotometry with diode-array detection. Fresenius’ Zeitschrift Für Analytische Chemie, 330(6), 510–515. https://doi.org/10.1007/BF00490759
Sundar, S., & Chakravarty, J. (2010). Antimony toxicity. International Journal of Environmental Research and Public Health, 7(12), 4267–4277. https://doi.org/10.3390/ijerph7124267
Tighe, M., Edwards, M. M., Cluley, G., Lisle, L., & Wilson, S. C. (2018). Colorimetrically determining total antimony in contaminated waters and screening for antimony speciation. Journal of Hydrology, 563, 84–91. https://doi.org/10.1016/j.jhydrol.2018.05.056
Tylenda, C.A., Tomei Torres, F.A., Sullivan, D.W. (2022). Chapter 2 - Antimony, in: G.F. Nordberg, M.B.T.-H. on the T. of M. (Fifth E. Costa (Eds.). Academic Press, Volume II, 23–40. https://doi.org/10.1016/B978-0-12-822946-0.00002-7.
Uluozlu, O. D., Sari, A., & Tuzen, M. (2010). Biosorption of antimony from aqueous solution by lichen (Physcia tribacia) biomass. Chemical Engineering Journal, 163(3), 382–388. https://doi.org/10.1016/j.cej.2010.08.022
Verma, N., Kaur, G. (2019). Chapter 8 - Advances in the oligonucleotide-based biosensors for the detection of heavy metal contaminants in the environment. Tools, Techniques and Protocols for Monitoring Environmental Contaminants, 169–185. https://doi.org/10.1016/B978-0-12-814679-8.00008-X
WHO. (2003). Antimony in drinking-water. https://cdn.who.int/media/docs/default-source/wash-documents/wash-chemicals/antimony.pdf?sfvrsn=e1e9a0a6_4
Yazıcı, E., Fırat, M., Selali Chormey, D., Bakırdere, E. G., & Bakırdere, S. (2020). An accurate determination method for cobalt in sage tea and cobalamin: Slotted quartz tube-flame atomic absorption spectrometry after preconcentration with switchable liquid-liquid microextraction using a Schiff base. Food Chemistry, 302, 125336. https://doi.org/10.1016/j.foodchem.2019.125336
Yıldırmaz, B. B., Gölcü, A., Zaman, B. T., Kasa, N. A., Bakırdere, E. G., & Bakırdere, S. (2021). Accurate and sensitive analytical method for trace iron determination in clove tea and tap water samples by slotted quartz tube-flame atomic absorption spectrometry after its preconcentration with supramolecular solvent-based liquid-phase microextraction. Chemical Papers, 75, 4157–4164. https://doi.org/10.1007/s11696-021-01652-5
Author information
Authors and Affiliations
Contributions
Determination of the method, experimental activities, interpretation of the analysis results, manuscript writing, and all other processes within the scope of this study were carried out by O. Y. The author read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval
Author has read, understood, and complied as applicable with the statement on "Ethical responsibilities of Authors" as found in the Instructions for Authors and is aware that with minor exceptions, no changes can be made to authorship once the paper is submitted
Competing interests
The author declares no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yağmuroğlu, O. Accurate and sensitive determination of Sb(III) in water samples using UV–VIS spectrophotometry after simultaneous complexation and preconcentration with deep eutectic solvent/DTZ probe-based liquid–liquid microextraction. Environ Monit Assess 195, 191 (2023). https://doi.org/10.1007/s10661-022-10809-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-022-10809-y