Abstract
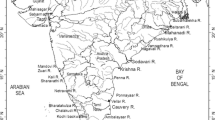
Mangrove sediments are known sources for methane emission that has a very high global warming potential. The spatio-temporal emission of methane in the mangrove sediments was quantified in the present study using the static closed chamber technique. Besides, the effects of environmental parameters on methane emission were estimated at Betim (mouth), Chorão (midstream), and Volvoi (upstream) stations along the tropical Mandovi estuary. On an average, the methane emission at the upstream estuarine station at Volvoi was maximum (1268.68 ± 176 nM cm−2 h−1) compared to the other two stations. Annually, the methane emission was significantly influenced by physicochemical parameters like salinity at Betim and Volvoi and, the redox potential at the midstream station at Chorão. The variation of methane emission between the 3 stations (P < 0.001) is attributed to the variation in methanotrophy (P < 0.05) and methanogenesis (P < 0.05) influenced by differences in the concentration of nutrients (P < 0.05) and organic carbon (P < 0.05). Seasonally, the highest methane emission at Chorão was during the post-monsoon, at Betim was during the monsoon season (1305.34 ± 108.58 nM cm−2 h−1), and at the upstream station at Volvoi, the emission of methane was highest during the pre-monsoon season (1514.68 ± 130.94 nM cm−2 h−1). The influence of environmental parameters was more prominent on methane emission at the 3 stations during the monsoon season. Spearman’s correlation analysis indicated that seasonal changes in methane emission are not only attributed to the influence of seasonal rainfall that leads to the fresh water input, but also to the variation in biogeochemical parameters.



Similar content being viewed by others
References
Al‐Haj, A. N., & Fulweiler, R. W. (2020). A synthesis of methane emissions from shallow vegetated coastal ecosystems. Global Change Biology, 26(5), 2988–3005. https://doi.org/10.1111/gcb.15046
Abril, G., & Borges, A.V. (2005). Carbon dioxide and methane emissions from estuaries. In Greenhouse gas emissions–fluxes and processes. (187–207) Springer, Berlin, Heidelberg.
Allen, D. E., Dalal, R. C., Rennenberg, H., Meyer, R. L., Reeves, S., & Schmidt, S. (2007). Spatial and temporal variation of nitrous oxide and methane flux between subtropical mangrove sediments and the atmosphere. Soil Biology and Biochemistry, 39(20), 622–631. https://doi.org/10.1016/j.soilbio.2006.09.013.
Allen, D., Dalal, R. C., Rennenberg, H., & Schmidt, S. (2011). Seasonal variation in nitrous oxide and methane emissions from subtropical estuary and coastal mangrove sediments, Australia. Plant Biology, 13(1), 126–133. https://doi.org/10.1111/j.1438-8677.2010.00331.x.
Alongi, D.M. (2007). The contribution of mangrove ecosystems to global carbon cycling and greenhouse gas emissions. Greenhouse gas and carbon balances in mangrove coastal ecosystems. Maruzen, Tokyo, 110.
Araujo, J., Naqvi, S. W. A., Naik, H., & Naik, R. (2018). Biogeochemistry of methane in a tropical monsoonal estuarine system along the west coast of India. Estuarine, Coastal and Shelf Science, 207, 435–443. https://doi.org/10.1016/j.ecss.2017.07.016
Bardgett, R. D., Freeman, C., & Ostle, N. J. (2008). Microbial contributions to climate change through carbon cycle feedbacks. The ISME Journal, 2(8), 805–814. https://doi.org/10.1038/ismej.2008.58
Bastviken, D., Ejlertsson, J., & Tranvik, L. (2002). Measurement of methane oxidation in lakes–A comparison of methods. Environmental Science and Technology, 36, 3354–3361. https://doi.org/10.1021/es010311p
Bligh, E. G., & Dyer, W. J. (1959). A rapid method for total lipid extraction and purification. Canadian Journal of Applied Physiology, 37, 911–917. https://doi.org/10.1139/o59-099
Biswas, H., Mukhopadhyay, S. K., Sen, S., & Jana, T. K. (2007). Spatial and temporal patterns of methane dynamics in the tropical mangrove dominated estuary, NE coast of Bay of Bengal, India. Journal of Marine Systems, 68(1–2), 55–64. https://doi.org/10.1016/j.jmarsys.2006.11.001.
Borrel, G., Jézéquel, D., Biderre-Petit, C., Morel-Desrosiers, N., Morel, J. P., Peyret, P., ... & Lehours, A. C. (2011). Production and consumption of methane in freshwater lake ecosystems. Research in microbiology, 162(9), 832–847. https://doi.org/10.1016/j.resmic.2011.06.004
Bouillon, S., Middelburg, J. J., Dehairs, F., Borges, A. V., Abril, G., Flindt, M. R., Ulomi, S., & Kristensen, E. (2007). Importance of intertidal sediment processes and porewater exchange on the water column biogeochemistry in a pristine mangrove creek (Ras Dege, Tanzania). Biogeosciences, 4(3), 311–322.
Bridgman, M. J., Lomax, B. H., & Sjögersten, S. (2020). Impacts of elevated atmospheric CO2 and Plant species composition on methane emissions from subarctic wetlands. Wetlands, 40(3), 609–618. https://doi.org/10.1007/s13157-019-01203-5
Capone, D. G., & Kiene, R. P. (1988). Comparison of microbial dynamics in marine and freshwater sediments: contrasts in anaerobic carbon catabolism. Limnol Oceanogr, 33:725–749. https://doi.org/10.4319/lo.1988.33.4part2.0725
Chauhan, R., Datta, A., Ramanathan, A. L., & Adhya, T. K. (2015). Factors influencing spatio-temporal variation of methane and nitrous oxide emission from a tropical mangrove of eastern coast of India. Atmospheric Environment, 107, 95–106. https://doi.org/10.1016/j.atmosenv.2015.02.006
Chen, G. C., Tam, N. F. Y., & Ye, Y. (2010). Summer fluxes of atmospheric greenhouse gases N2O, CH4 and CO2 from mangrove soil in South China. Science of the Total Environment, 408(13), 2761–2767. https://doi.org/10.1016/j.scitotenv.2010.03.007
Chen, G. C., Tam, N. F. Y., Wong, Y. S., & Ye, Y. (2011). Effect of wastewater discharge on greenhouse gas fluxes from mangrove soils. Atmospheric Environment, 45(5), 1110–1115. https://doi.org/10.1016/j.atmosenv.2010.11.034
Chen, G. C., Ulumuddin, Y. I., Pramudji, S., Chen, S. Y., Chen, B., Ye, Y., Ou, D. Y., Ma, Z. Y., Huang, H., & Wang, J. K. (2014). Rich soil carbon and nitrogen but low atmospheric greenhouse gas fluxes from North Sulawesi mangrove swamps in Indonesia. Science of the Total Environment, 487, 91–96. https://doi.org/10.1016/j.scitotenv.2014.03.140
Chmura, G. L., Anisfeld, S. C., Cahoon, D. R., & Lynch, J. C. (2003). Global carbon sequestration in tidal, saline wetland soils. Global Biogeochemical Cycles, 17, 1111. https://doi.org/10.1029/2002GB001917.
Conrad, R. (2009). The global methane cycle: Recent advances in understanding the microbial processes involved. Environmental Microbiology Reports, 1(5), 285–292. https://doi.org/10.1111/j.1758-2229.2009.00038.x.
Corman, J. R., Bertolet, B. L., Casson, N. J., Sebestyen, S. D., Kolka, R. K., & Stanley, E. H. (2018). Nitrogen and phosphorus loads to temperate seepage lakes associated with allochthonous dissolved organic carbon loads. Geophysical Research Letters, 45, 5481–5490. https://doi.org/10.1029/2018GL077219
Danovaro, R., Fabiano, M., & Della Croce, N. (1993). Labile organic matter and microbial biomasses in deep-sea sediments (Eastern Mediterranean Sea). Deep Sea Research Part I: Oceanographic Research Papers, 40, 953–965. https://doi.org/10.1016/j.marpolbul.2005.09.027.
De Sousa, S. N., Gupta, R. S., Sanzgiri, S., & Rajagopal, M. D. (1981). Studies on Nutrients of Mandovi & Zuari River Systems.
Dutta, M. K., Bianchi, T. S., & Mukhopadhyay, S. K. (2017). Mangrove methane biogeochemistry in the Indian Sundarbans: A proposed budget. Frontiers in Marine Science, 4, 187. https://doi.org/10.3389/fmars.2017.00187
Divya, B., Fernandes, S. O., Sheelu, G., Nair, S., Bharathi, P. L., & Chandramohan, D. (2009). Limno-tolerant bacteria govern nitrate concentration in Mandovi Estuary, India. Estuarine, Coastal and Shelf Science, 82(1), 29–34. https://doi.org/10.1016/j.ecss.2008.11.020.
Duc, N. T., Crill, P., & Bastviken, D. (2010). Implications of temperature and sediment characteristics on methane formation and oxidation in lake sediments. Biogeochemistry, 100(1), 185–196. https://doi.org/10.1007/s10533-010-9415-8
Egger, M., Lenstra, W., Jong, D., Meysman, F. J., Sapart, C. J., Van der Veen, C., & Slomp, C. P. (2016). Rapid sediment accumulation results in high methane effluxes from coastal sediments. PLoS One, 11(8), e0161609. https://doi.org/10.1371/journal.pone.0161609
El Wakeel, S. K., & Riley, J. P. (1957). Determination of organic carbon in the marine muds. Journal Du Conseil Intrenational Pour L’exploration De La Mer, 22, 180–183. https://doi.org/10.1093/icesjms/22.2.180.
Fernandes, S. O., Bharathi, P. A., Bonin, P. C., & Michotey, V. D. (2010). Denitrification: An important pathway for nitrous oxide production in tropical mangrove sediments (Goa, India). Journal of Environmental Quality, 39(4), 1507–1516. https://doi.org/10.2134/jeq2009.0477
Flanagan, L. B., & Syed, K. H. (2011). Stimulation of both photosynthesis and respiration in response to warmer and drier conditions in a boreal peatland ecosystem. Global Change Biology, 17, 2271–2287. https://doi.org/10.1111/j.1365-2486.2010.02378.x
Gazeau, F., Smith, S. V., Gentili, B., Frankignoulle, M., & Gattuso, J. P. (2004). The European coastal zone: Characterization and first assessment of ecosystem metabolism. Estuarine, Coastal and Shelf Science, 60(4), 673–694. https://doi.org/10.1016/j.ecss.2004.03.007.
Giani, L., Bashan, Y., Holguin, G., & Strangmann, A. (1996). Characteristics and methanogenesis of the Balandra lagoon mangrove soils, Baja California Sur, Mexico. Geoderma, 72(1–2), 149–160. https://doi.org/10.1016/0016-7061(96)00023-7.
Gonsalves, M. J., Fernandes, C. E. G., Fernandes, S. O., Kirchman, D. L., & Loka Bharathi, P. A. (2011). Effects of composition of labile organic matter on biogenic production of methane in the coastal sediments of the Arabian Sea. Environmental Monitoring and Assessment, 182, 385–395. https://doi.org/10.1007/s10661-011-1883-3
Gonsalves, M. J., Nair, S., LokaBharathi, P. A., & Chandraohan, D. (2009). Abundance and production of particle-associated bacteria and their role in a mangrove-dominated estuary. Aquatic Microbial Ecology, 57, 151–159. https://doi.org/10.3354/ame01337
Harmsen, M., van Vuuren, D. P., Bodirsky, B. L., Chateau, J., Durand-Lasserve, O., Drouet, L., Fricko, O., Fujimori, F., Gernaat, D., Hanaoka, T., Hilaire, J., Keramidas, K., Luderer, G., Moura, M., Sano, F., Smith, S., & Wada, K. (2020). The role of methane in future climate strategies: Mitigation potentials and climate impacts. Climate Change, 163(3), 1409–1425. https://doi.org/10.1007/s10584-019-02437-2
Hinrichs, K. U., Summons, R. E., Orphan, V., Sylva, S. P., & Hayes, J. M. (2000). Molecular and isotopic analysis of anaerobic methane-oxidizing communities in marine sediments. Organic Geochemistry, 31(12), 1685–1701. https://doi.org/10.1016/S0146-6380(00)00106-6
Inubushi, K., Furukawa, Y., Hadi, A., Purnomo, E., & Tsuruta, H. (2003). Seasonal changes of CO2, CH4 and N2O fluxes in relation to land-use change in tropical peatlands located in coastal area of South Kalimantan. Chemosphere, 52(3), 603–608. https://doi.org/10.1016/S0045-6535(03)00242-X
Intergovernmental Panel on climate change. (IPCC, 2021). https://www.ipcc.ch/2021/08/09/ar6-wg1-20210809-pr/
Jagtap, T. G., Chavan, V. S., & Untawale, A. G. (1993). Mangrove Ecosystems of India: A need for protection (synopsis). Ambio, 22, 252–254.
Jagtap, T. G., Bhosale, S., & Singh, C. (2006). Characterisation of Porteresia coarctata beds along the Goa coast, India. Aquatic Botany, 84, 37–44. https://doi.org/10.1016/j.aquabot.2005.07.010
Jayakumar, D. A., Naqvi, S. W. A., Narvekar, P. V., & George, M. D. (2001). Methane in coastal and offshore waters of the Arabian Sea. Marine Chemistry, 74(1), 1–13. https://doi.org/10.1016/S0304-4203(00)00089-X
Kamaleson, A. S., & Gonsalves, M. J. (2019). Role of sulfur-oxidizing bacteria on the ecology in tropical mangrove sediments. Regional Studies in Marine Science, 28, 100574. https://doi.org/10.1016/j.rsma.2019.100574
Kathiresan, K., & Rajendran, N. (2005). Coastal mangrove forests mitigated tsunami. Estuarine, Coastal and shelf science, 65(3), 601–606. https://doi.org/10.1016/j.ecss.2005.06.022
Kathiresan, K., Gomathi, V., Anburaj, R., & Saravanakumar, K. (2014). Impact of mangrove vegetation on seasonal carbon burial and other sediment characteristics in the Vellar-Coleroon estuary, India. Journal of forestry research, 25(4), 787–794. https://doi.org/10.1007/s11676-014-0526-2
Knittel, K., & Boetius, A. (2009). Anaerobic oxidation of methane: progress with an unknown process. Annual review of microbiology, 63, 311–334. https://doi.org/10.1146/annurev.micro.61.080706.093130
Kochert, A. G. (1978). Carbohydrate determination by the phenol–sulfuric acid method. In J. A. Hellebust & J. S. Craigie (Eds.), Handbook of phycological methods: Physiological and biochemical methods (pp. 95–97). Cambridge University Press.
Kreuzwieser, J., Buchholz, J., & Rennenberg, H. (2003). Emission of methane and nitrous oxide by Australian mangrove ecosystems. Plant Biology, 5(4), 423–431. https://doi.org/10.1055/s-2003-42712
Krithika, K., Purvaja, R., & Ramesh, R. (2008). Fluxes of methane and nitrous oxide from an Indian mangrove. Current Science, 94(2), 218–224.
Kristensen, E., Flindt, M. R., Ulomi, S., Borges, A. V., Abril, G., & Bouillon, S. (2008). Emission of CO2 and CH4 to the atmosphere by sediments and open waters in two Tanzanian mangrove forests. Marine Ecology Progress Series, 370, 53–67. https://doi.org/10.3354/meps07642
Krupadam, R. J., Ahuja, R., Wate, S. R., & Anjaneyulu, Y. (2007). Forest bound estuaries are higher methane emitters than paddy fields: A case of Godavari estuary. East Coast of India. Atmos. Environ, 41(23), 4819–4827. https://doi.org/10.1016/j.atmosenv.2007.02.023
Kumar, G., & Ramanathan, A. L. (2014). Biogeochemistry of methane emission in mangrove ecosystem–review.
Laiho, R. (2006). Decomposition in peatlands: Reconciling seemingly contrasting results on the impacts of lowered water levels. Soil Biology & Biochemistry, 38, 2011–2024. https://doi.org/10.1016/j.soilbio.2006.02.017.
Le Mer, J., & Roger, P. (2001). Production, oxidation, emission and consumption of methane by soils: a review. European journal of soil biology, 37(1), 25–50. https://doi.org/10.1016/S1164-5563(01)01067-6
Lin, C. W., Kao, Y. C., Chou, M. C., Wu, H. H., Ho, C. W., & Lin, H. J. (2020). Methane emissions from subtropical and tropical mangrove ecosystems in Taiwan. Forests, 11(4), 470. https://doi.org/10.3390/f11040470
Liu, J., Zhou, Y., Valach, A., Shortt, R., Kasak, K., Rey-Sanchez, C., Hemes, K. S., Baldocchi, D., & Lai, D. Y. (2020). Methane emissions reduce the radiative cooling effect of a subtropical estuarine mangrove wetland by half. Global Change Biology, 26(9), 4998–5016. https://doi.org/10.1111/gcb.15247
Livesley, S. J., & Andrusiak, S. M. (2012). Temperate mangrove and salt marsh sediments are a small methane and nitrous oxide source but important carbon store. Estuarine, Coastal and Shelf Science, 97, 19–27. https://doi.org/10.1016/j.ecss.2011.11.002
Lowry, O. H., Rosebrough, N. J., Farr, A. L., & Randall, R. (1951). Protein measurement with the Folin phenol reagent. Journal of Biological Chemistry, 193, 265–275. https://doi.org/10.1016/S0021-9258(19)52451-6
Luo, M., Huang, J. F., Zhu, W. F., & Tong, C. (2019). Impacts of increasing salinity and inundation on rates and pathways of organic carbon mineralization in tidal wetlands: a review. Hydrobiologia, 827(1), 31–49. https://doi.org/10.1007/s10750-017-3416-8
Marinho, C. C., Campos, E. A., Guimarães, J. R. D., & Esteves, F. A. (2012). Effect of sediment composition on methane concentration and production in the transition zone of a mangrove (Sepetiba Bay, Rio de Janeiro, Brazil). Brazilian Journal of Biology, 72(3), 429–436. https://doi.org/10.1590/S1519-69842012000300003
Martens, C. S., & Klump, J. V. (1984). Biogeochemical cycling in an organic-rich coastal marine basin 4. An organic carbon budget for sediments dominated by sulfate reduction and methanogenesis. Geochimica et Cosmochimica Acta, 48(10): 1987–2004. https://doi.org/10.1016/0016-7037(84)90380-6
Martens, C. S., Albert, D. B., & Alperin, M. J. (1998). Biogeochemical processes controlling methane in gassy coastal sediments–Part 1. A model coupling organic matter flux to gas production, oxidation and transport. Continental Shelf Research, 18(14–15): 1741–1770. https://doi.org/10.1016/S0278-4343(98)00056-9
Meulepas, R. J., Jagersma, C. G., Khadem, A. F., Stams, A. J., & Lens, P. N. (2010). Effect of methanogenic substrates on anaerobic oxidation of methane and sulfate reduction by anaerobic methanotrophic enrichment. Applied Microbiology and Biotechnology, 87(4), 1499–1506. https://doi.org/10.1007/s00253-010-2597-0.
Muñoz, H. M., Morell, J. M., & Corredor, J. E. (2002). Increase of nitrous oxide flux to the atmosphere upon nitrogen addition to red mangroves sediments. Marine Pollution Bulletin, 44(10), 992–996. https://doi.org/10.1016/S0025-326X(02)00132-7
Myhre, G., Shindell, D., Bréon, F. M., Collins, W., Fuglestvedt, J., Huang, J., Koch, D., Lamarque, J. F., Lee, D., Mendoza, B., & Nakajima, T. (2013). Anthropogenic and natural radiative forcing, climate change 2013: The physical science basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change 659–740.
Naik, R., Araujo, J., Pratihary, A., Kurian, S., & Naqvi, S. W. A. (2019). Sedimentary sulphate reduction and organic matter mineralization across salinity gradient of the Mandovi Estuary, West coast of India. Estuarine, Coastal and Shelf Science, 221, 21–29. https://doi.org/10.1016/j.ecss.2019.03.005
Parsons, T. R., Maita, Y., & Lalli, C. M. (1984). Amanual of chemical and biological methods for seawater analysis. In Biological Oceanographic Processes (p. 173). New York: Pergamon Press. https://doi.org/10.1016/C2009-0-07774-5
Prasad, M. B. K., & Ramanathan, A. L. (2008). Sedimentary nutrient dynamics in a tropical estuarine mangrove ecosystem. Estuarine, Coastal and Shelf Science, 80(1), 60–66. https://doi.org/10.1016/j.ecss.2008.07.004
Purvaja, R., & Ramesh, R. (2000). Human impacts on methane emission from mangrove ecosystems in India. Regional Environmental Change, 1(2), 86–97. https://doi.org/10.1007/PL00011537
Purvaja, R., & Ramesh, R. (2001). Natural and anthropogenic methane emission from coastal wetlands of South India. Environmental Management, 27(4), 547–557. https://doi.org/10.1007/s002670010169
Purvaja, R., Ramesh, R., & Frenzel, P. (2004). Plant-mediated methane emission from an Indian mangrove. Global Change Biology, 10, 1825–1834. https://doi.org/10.1111/j.1365-2486.2004.00834.x
Qasim, S. Z., & Gupta, R. S. (1981). Environmental characteristics of the Mandovi-Zuari estuarine system in Goa. Estuarine, Coastal and Shelf Science, 13(5), 557–578. https://doi.org/10.1016/S0302-3524(81)80058-8
Ramanathan, A.L. (1997). Sediment characteristics of the Pichavaram mangrove environment, southeast coast of India. Journal of marine science, 26, 319-322 .
Rao, G. D., & Sarma, V. V. S. S. (2016). Variability in concentrations and fluxes of methane in the Indian estuaries. Estuaries and Coasts, 39(6), 1639–1650. https://doi.org/10.1007/s12237-016-0112-2
Ray, R., Ganguly, D., Chowdhury, C., Dey, M., Das, S., Dutta, M. K., Mandal, S. K., Majumder, N., De, T. K., Mukhopadhyay, S. K., & Jana, T. K. (2011). Carbon sequestration and annual increase of carbon stock in a mangrove forest. Atmospheric Environment, 45(28), 5016–5024. https://doi.org/10.1016/j.atmosenv.2011.04.074
Reshmi, R. R., Nair, K. D., Zachariah, E. J., & Vincent, S. G. T. (2015). Methanogenesis: Seasonal changes in human impacted regions of Ashtamudi estuary (Kerala, South India). Estuarine, Coastal and Shelf Science, 156, 144–154. https://doi.org/10.1016/j.ecss.2014.11.031
Rosentreter, J. A., Borges, A. V., Deemer, B. R., Holgerson, M. A., Liu, S., Song, C., ... & Eyre, B. D. (2021). Half of global methane emissions come from highly variable aquatic ecosystem sources. Nature Geoscience, 14(4), 225–230. https://doi.org/10.1038/s41561-021-00715-2
Rosentreter, J. A., Maher, D. T., Erler, D. V., Murray, R. H., & Eyre, B. D. (2018). Methane emissions partially offset “blue carbon” burial in mangroves. Science advances, 4(6), eaao4985. https://doi.org/10.1038/s41561-021-00715-2
Rummasak, T., Towprayoon, S., & Bashkin, V. (2002). Chemical properties of mangrove sediments in relation to sulfur dynamics. Tropics, 12(1), 43–57.
Salimi, S., Almuktar, S. A., & Scholz, M. (2021). Impact of climate change on wetland ecosystems: A critical review of experimental wetlands. Journal of Environmental Management, 286, 112–160. https://doi.org/10.1016/j.jenvman.2021.112160
Sardessai, S., & Sundar, D. (2007). Variability of nitrate and phosphate. In S. R. Shetye, M. Dileep Kumar, & D. Shankar (Eds.), The Mandovi and Zuari estuaries (pp. 59–66). National Institute of Oceanography.
Schaeffer-Novelli, Y., Coelho Junior, C., & Tognella-De-Rosa, M. (2002). Manguezais. São Paulo: Ática. 48 p.
Schubert, C. J., Lucas, F. S., Durisch-Kaiser, E., Stierli, R., Diem, T., Scheidegger, O., Vazquez, F., & Müller, B. (2010). Oxidation and emission of methane in a monomictic lake (Rotsee, Switzerland). Aquatic Sciences, 72(4), 455–466. https://doi.org/10.1007/s00027-010-0148-5
Segarra, K. E., Comerford, C., Slaughter, J., & Joye, S. B. (2013). Impact of electron acceptor availability on the anaerobic oxidation of methane in coastal freshwater and brackish wetland sediments. Geochimica Et Cosmochimica Acta, 115, 15–30. https://doi.org/10.1016/j.gca.2013.03.029
Segers, R. (1998). Methane production and methane consumption: a review of processes underlying wetland methane fluxes. Biogeochemistry, 41(1), 23–51.
Singh, G., Ramanathan, A. L., & Prasad, M. B. K. (2005). Nutrient cycling in mangrove ecosystem: A brief overview. International Journal of Environmental Sciences, 30, 231–244.
Shynu, R., Rao, V. P., Sarma, V. V. S. S., Kessarkar, P. M., & ManiMurali, R. (2015). Sources and fate of organic matter in suspended and bottom sediments of the Mandovi and Zuari estuaries, western India.
Sotomayor, D., Corredor, J. E., & Morell, J. M. (1994). Methane flux from mangrove sediments along the southwestern coast of Puerto Rico. Estuaries, 17(1), 140–147. https://doi.org/10.2307/1352563
Stets, E. G., & Cotner, J. B. (2008). The influence of dissolved organic carbon on bacterial phosphorus uptake and bacteria-phytoplankton dynamics in two Minnesota lakes. Limnology and Oceanography, 53, 137–147. https://doi.org/10.4319/lo.2008.53.1.0137
Tishchenko, P., Hensen, C., Wallmann, K., & Wong, C. S. (2005). Calculation of the stability and solubility of methane hydrate in seawater. Chemical geology, 219(1-4), 373–52. https://doi.org/10.1016/j.chemgeo.2005.02.008
Tomlinson, P. B. (1994). The botany of mangroves. Cambridge: Cambridge University Press. 419. https://doi.org/10.1017/CBO9781139946575
Ulumuddin, Y. I., Beavis, S., Roderick, M., Eggins, S., Sugoro, I., & Sukardjo, S. (2021). Potential carbon loss in sediment through methane production during early development stage of mangrove regeneration in restored mangroves. In Dynamic Sedimentary Environments of Mangrove Coasts (pp. 415445). Elsevier. https://doi.org/10.1016/B978-0-12-816437-2.00020-3
Venturini, N., Pita, A. L., Brugnoli, E., García-Rodríguez, F., Burone, L., Kandratavicius, N., Hutton, M., & Muniz, P. (2012). Benthic trophic status of sediments in a metropolitan area (Rio de la Plata estuary): Linkages with natural and human pressures. Estuarine, Coastal and Shelf Science, 112, 139–152. https://doi.org/10.1016/j.ecss.2011.08.016
Wang, H., Liao, G., D’Souza, M., Yu, X., Yang, J., Yang, X., & Zheng, T. (2016). Temporal and spatial variations of greenhouse gas fluxes from a tidal mangrove wetland in Southeast China. Environmental Science and Pollution Research, 23(2), 1873–1885. https://doi.org/10.1007/s11356-015-5440-4
Wafar, S. (1987). Ecology of mangroves along the estuaries of Goa. Ph.D. Thesis, University of Karnataka, India, 299 pp.
Wafar, S., Untawale, A. G., & Wafar, M. (1997). Litter fall and energy flux in a mangrove ecosystem. Estuarine, Coastal and Shelf Science, 44(1), 111–124. https://doi.org/10.1006/ecss.1996.0152
Walter, B. P., & Heimann, M. (2000). A process-based, climate-sensitive model to derive methane emissions from natural wetlands: Application to five wetland sites, sensitivity to model parameters, and climate. Global Biogeochemical Cycles, 14(3), 745–765. https://doi.org/10.1029/1999GB001204
Yavitt, J. B., & Knapp, A. K. (1995). Methane emission to the atmosphere through emergent cattail (Typha latifolia L.) plants. Tellus B: Chemical and Physical Meteorology, 47(5), 521–534. https://doi.org/10.3402/TELLUSB.V47I5.16065
Yu, K., Faulkner, S. P., & Patrick Jr, W. H. (2006). Redox potential characterization and soil greenhouse gas concentration across a hydrological gradient in a Gulf coast forest. Chemosphere, 62(6), 905–914. https://doi.org/10.1016/j.chemosphere.2005.05.033
Zhang, Z., Zimmermann, N. E., Stenke, A., Li, X., Hodson, E. L., Zhu, G., et al. (2017). Emerging role of wetland methane emissions in driving 21st century climate change. Proceedings of the National Academy of Sciences, 114(36), 9647–9652. https://doi.org/10.1073/pnas.1618765114.
Zheng, X., Guo, J., Song, W., Feng, J., & Lin, G. (2018). Methane emission from mangrove wetland soils is marginal but can be stimulated significantly by anthropogenic activities. Forests, 9(12), 738. https://doi.org/10.3390/f9120738
Acknowledgements
The authors are grateful to the Director CSIR-NIO. This study is a part of the project on the “Functional role of methanotrophs in mangrove sediments” (Ref No: SR/S4/ES-651/2012) sponsored by Department of Science and Technology, New Delhi, India. The authors thank the Forest Department, Goa, for permitting to carry out the on field experiment in the Mangrove Ecosystem. This is NIO contribution no.6856
Funding
Department of Science and Technology, New Delhi, India.
Author information
Authors and Affiliations
Contributions
(1) Delcy R. Nazareth – sampling, experimental work, data analysis and manuscript writing. (2) Maria Judith Gonsalves – conceptualising the work, data validation, explication of data, finalisation of manuscript and project leader
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nazareth, D.R., Gonsalves, MJ. Influence of seasonal and environmental variables on the emission of methane from the mangrove sediments of Goa. Environ Monit Assess 194, 249 (2022). https://doi.org/10.1007/s10661-021-09734-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-021-09734-3