Abstract
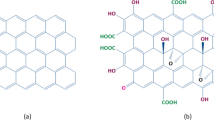
The adsorption of sulfur-containing compounds from fuels on polymer nanocomposite adsorbents modified with carbon-based materials was investigated. This work reports the removal of benzothiophene from the model liquid fuel using a novel adsorbent prepared by nanopoly (4,4′-methylenedianiline)-graphene oxide (NPMDA/GO) composite. The adsorbent was successfully synthesized with the in situ electrochemical method and characterized using thermal gravimetric analysis (TGA), field-scattering scanning electron microscopy (FE-SEM), transient electron microscopy (TEM), X-ray diffraction analysis (XRD), and Fourier transform infrared (FTIR). The results showed that the presence of graphene oxide (GO) nanosheets in NPMDA/GO composite adsorbent due to its high tendency to sulfur would increase the adsorption of benzothiophene compared to unmodified nanopoly (4,4′-methylenedianiline) (NPMDA). The π_complexation, oxygenated organic functional groups, and layered sheets of graphene oxide improve adsorption capacity for desulfurization. The NPMDA/GO composite presented maximum efficiency (63.33%) at 30 mg/L initial concentration, 120 mg adsorbent dose, and 120 min contact time at 25 °C. Furthermore, the adsorbent shows an almost good reusability after four cycles (67.12 mg/g sulfur absorption capacity). Pseudo-second-order model (R2 = 0.9975) and the Freundlich isotherm (R2 = 0.8813) were used to describe the adsorption process. Findings confirm that NPMDA/GO composite can be applicable for removal of benzothiophene from liquid fuels.














Similar content being viewed by others
Data availability
All necessary data are present within the manuscript.
References
Abdelhafez, A. A., & Li, J. (2016). Removal of Pb (II) from aqueous solution by using biochars derived from sugar cane bagasse and orange peel. Journal of the Taiwan Institute of Chemical Engineers, 61, 367–375
Ahmad, S., Ahmad, M. I., Naeem, K., Humayun, M., & Sebt-E-Zaeem, F. F. (2016). Oxidative desulfurization of tirepyrolysis oil. Chemical Industry and Chemical Engineering Quarterly, 22, 249–254
Basu, H., Saha, S., Kailasa, S. K., & Singhal, R. K. (2020). Present status of hybrid materials for potable water decontamination: A review. Environmental Science: Water Research & Technology, 6(12), 3214–3248
Chen, M. M. (2016). Thermal analysis. Materials Science and Engineering of Carbon, 249–272. Butterworth-Heinemann
Díaz, I. G., Alguacil, F. J., Escudero, E., & López, F. A. (2020). Evaluation of La (III) and Ce (III) Adsorption from aqueous solution using carbon nanotubes adsorbent
Długosz, O., & Banach, M. (2018). Kinetic, isotherm and thermodynamic investigations of the adsorption of Ag+ and Cu2+ on vermiculite. Journal of Molecular Liquids, 258, 295–309.
Duan, F., Chen, C., Wang, G., Yang, Y., Liu, X., & Qin, Y. (2014). Efficient adsorptive removal of dibenzothiophene by graphene oxide-based surface molecularly imprinted polymer. RSC Advances, 4(3), 1469–1475.
Fadillah, G., Saleh, T. A., Wahyuningsih, S., Putri, E. N. K., & Febrianastuti, S. (2019). Electrochemical removal of methylene blue using alginate-modified graphene adsorbents. Chemical Engineering Journal, 378, 122140
Fu, S., Sun, Z., Huang, P., Li, Y., & Hu, N. (2019). Some basic aspects of polymer nanocomposites: A critical review. Nano Materials Science, 1(1), 2–30
Giżyński, M., & Romelczyk-Baishya, B. (2021). Investigation of carbon fiber–reinforced thermoplastic polymers using thermogravimetric analysis. Journal of Thermoplastic Composite Materials, 34(1), 126–140
Hassan, H. K., Atta, N. F., & Galal, A. (2012). Electropolymerization of aniline over chemically converted graphene-systematic study and effect of dopant. International Journal of Electrochemical Science, 7, 11161–11181
Hernández-Maldonado, A. J., & Yang, R. T. (2004). New sorbents for desulfurization of diesel fuels via π-complexation. AIChE Journal, 50(4), 791–801
Hu, T. P., Zhang, Y. M., Zheng, L. H., & Fan, G. Z. (2010). Molecular recognition and adsorption performance of benzothiophene imprinted polymer on silica gel surface. Journal of Fuel Chemistry and Technology, 38(6), 722–729
Kaur, S., Rani, S., & Mahajan, R. K. (2013). Adsorption kinetics for the removal of hazardous dye congo red by biowaste materials as adsorbents. Journal of Chemistry
Khan, N. A., & Jhung, S. H. (2012a). Adsorptive removal of benzothiophene using porous copper-benzenetricarboxylate loaded with phosphotungstic acid. Fuel Processing Technology, 100, 49–54
Khan, N. A., & Jhung, S. H. (2012b). Remarkable adsorption capacity of CuCl2-loaded porous vanadium benzenedicarboxylate for benzothiophene. Angewandte Chemie International Edition, 51(5), 1198–1201
Khan, N. A., Bhadra, B. N., & Jhung, S. H. (2018). Heteropoly acid-loaded ionic liquid@ metal-organic frameworks: Effective and reusable adsorbents for the desulfurization of a liquid model fuel. Chemical Engineering Journal, 334, 2215–2221
Lee, K. X., & Valla, J. A. (2017). Investigation of metal-exchanged mesoporous Y zeolites for the adsorptive desulfurization of liquid fuels. Applied Catalysis b: Environmental, 201, 359–369
Li, Y., Zheng, J. L., Feng, J., & Jing, X. L. (2013). Polyaniline micro-/nanostructures: Morphology control and formation mechanism exploration. Chemical Papers, 67(8), 876–890
Liang, J., Huang, Y., Zhang, L., Wang, Y., Ma, Y., Guo, T., & Chen, Y. (2009). Molecular-level dispersion of graphene into poly (vinyl alcohol) and effective reinforcement of their nanocomposites. Advanced Functional Materials, 19(14), 2297–2302
Liu, B., Vikrant, K., Kim, K. H., Kumar, V., & Kailasa, S. K. (2020). Critical role of water stability in metal–organic frameworks and advanced modification strategies for the extension of their applicability. Environmental Science: Nano, 7(5), 1319–1347
Liu, H., & Qiu, H. (2020). Recent advances of 3D graphene-based adsorbents for sample preparation of water pollutants: A review. Chemical Engineering Journal, 393, 124691
Liu, W., Liu, X., Yang, Y., Zhang, Y., & Xu, B. (2014). Selective removal of benzothiophene and dibenzothiophene from gasoline using double-template molecularly imprinted polymers on the surface of carbon microspheres. Fuel, 117, 184–190
Lu, H., Zhang, S., Guo, L., & Li, W. (2017). Applications of graphene-based composite hydrogels: A review. RSC Advances, 7(80), 51008–51020
Mark, J. E. (2009). Polymer Data Handbook, Oxford University
Mashkoor, F., & Nasar, A. (2020). Carbon nanotube-based adsorbents for the removal of dyes from waters: A review. Environmental Chemistry Letters, 18(3), 605–629
Matloob, A. M., Abd El-Hafiz, D. R., Saad, L., Mikhail, S., & Guirguis, D. (2019). Metal organic framework-graphene nano-composites for high adsorption removal of DBT as hazard material in liquid fuel. Journal of Hazardous Materials, 373, 447–458
Mohammad, S. G., Ahmed, S. M., Amr, A. E. G. E., & Kamel, A. H. (2020). Porous activated carbon from lignocellulosic agricultural waste for the removal of acetampirid pesticide from aqueous solutions. Molecules, 25(10), 2339
Mohseni, E., Hamdi, Z., Parvizimehr, A., & Rahmani, A. (2021). Adsorptive desulphurisation of benzothiophene and dibenzothiophene from model fuels with modified vermiculite. International Journal of Environmental Analytical Chemistry, 1–15. https://doi.org/10.1080/03067319.2021.1942461
Mohseni, E., Yaftian, M.R., Shayani-jam, H., Zamani, A., & Piri, F. (2020). Molecularly imprinted poly (4, 4′-methylenedianiline) as electrochemical sensor for determination of 1-benzothiophene. Synthetic Metals, 259, 116252
Montazeri, S. M., & Sadrnezhaad, S. K. (2019). Kinetics of sulfur removal from Tehran vehicular gasoline by g-C3N4/SnO2 nanocomposite. ACS Omega, 4(8), 13180–13188
Nuntang, S., Prasassarakich, P., & Ngamcharussrivichai, C. (2008). Comparative study on adsorptive removal of thiophenic sulfurs over Y and USY zeolites. Industrial & Engineering Chemistry Research, 47(19), 7405–7413
Palomino, J. M., Tran, D. T., Kareh, A. R., Miller, C. A., Gardner, J. M., Dong, H., & Oliver, S. R. (2015). Zirconia-silica based mesoporous desulfurization adsorbents. Journal of Power Sources, 278, 141–148
Paszkiewicz, S., & Szymczyk, A. (2019). Graphene-based nanomaterials and their polymer nanocomposites. In Nanomaterials and polymer nanocomposites, 177–216. Elsevier
Polini, A., & Yang, F. (2017). Physicochemical characterization of nanofiber composites. In Nanofiber composites for biomedical applications, 97–115. Woodhead Publishing
Qiu, J., Wang, G., Bao, Y., Zeng, D., & Chen, Y. (2015). Effect of oxidative modification of coal tar pitch-based mesoporous activated carbon on the adsorption of benzothiophene and dibenzothiophene. Fuel Processing Technology, 129, 85–90
Rai, P. K., Lee, J., Kailasa, S. K., Kwon, E. E., Tsang, Y. F., Ok, Y. S., & Kim, K. H. (2018). A critical review of ferrate (VI)-based remediation of soil and groundwater. Environmental Research, 160, 420–448
Saleh, T. A., Sulaiman, K. O., & AL-Hammadi, S.A., Dafalla, H. and Danmaliki, G.I., . (2017). Adsorptive desulfurization of thiophene, benzothiophene and dibenzothiophene over activated carbon manganese oxide nanocomposite: With column system evaluation. Journal of Cleaner Production, 154, 401–412
Sekar, R., Kailasa, S. K., Li, W. S., Wu, H. C., & Wu, H. F. (2013). Rapid separation of acetophenone and its monohydroxy isomers by capillary electrophoresis. Chinese Chemical Letters, 24(9), 833–836
Shahabuddin, S., Sarih, N. M., Afzal Kamboh, M., Rashidi Nodeh, H., & Mohamad, S. H. (2016). Synthesis of polyaniline-coated graphene oxide@SrTiO3 nanocube nanocomposites for enhanced removal of carcinogenic dyes from aqueous solution. Polymers, 8(9), 305
Sharif, F., & Roberts, E. P. (2020). Anodic electrochemical regeneration of a graphene/titanium dioxide composite adsorbent loaded with an organic dye. Chemosphere, 241, 125020
Simonin, J. P. (2016). On the comparison of pseudo-first order and pseudo-second order rate laws in the modeling of adsorption kinetics. Chemical Engineering Journal, 300, 254–263
Sun, R., Li, L., Feng, C., Kitipornchai, S., & Yang, J. (2018). Tensile behavior of polymer nanocomposite reinforced with graphene containing defects. European Polymer Journal, 98, 475–482
Sun, X., Huang, C., Wang, L., Liang, L., Cheng, Y., Fei, W., & Li, Y. (2021). Recent progress in graphene/polymer nanocomposites. Advanced Materials, 33(6), 2001105
Toolabi, A., Mohseni, E., Zare, M. R., Mengelizadeh, N., Rostami, E., Taghavig, M., & Kharazi, S. (2021). Effective removal of Diazinon and Imidacloprid toxins from aqueous samples by nano poly (4,4′-methylenedianiline) / graphene oxide. Desalination and Water Treatment. https://doi.org/10.5004/dwt.2021.27516
Ullah, L., Zhao, G., Hedin, N., Ding, X., Zhang, S., Yao, X., Nie, Y., & Zhang, Y. (2019). Highly efficient adsorption of benzothiophene from model fuel on a metal-organic framework modified with dodeca-tungstophosphoric acid. Chemical Engineering Journal, 362, 30–40
Yang, L., Wang, Y., Huang, D., Luo, G., & Dai, Y. (2007). Preparation of high performance adsorbents by functionalizing mesostructured silica spheres for selective adsorption of organosulfur compounds. Industrial & Engineering Chemistry Research, 46(2), 579–583
Zamanian, Z., Yousefinejad, S., Khoshnoud, M. J., Golbabaie, F., Farhang Dehghan, S., Modaresi, A., Amanat, S., Reza Zare, M., & Rahmani, A. (2018). Toxic effects of subacute inhalation exposure to trichloroethylene on serum lipid profile, glucose and biochemical parameters in Sprague-Dawley rats. Inhalation Toxicology, 30(9–10), 354–360
Zamanian, Z., Yousefinejad, S., Khoshnoud, M. J., Golbabaie, F., Zare, M. R., Modaresi, A., Zarei, M. R., & Rahmani, A. (2019). Toxicological effects of inhalation exposure to trichloroethylene on serum immunoglobulin and electrolyte levels in rats. Health Scope, 8(3)
Zhang, K., Zhang, L. L., Zhao, X. S., & Wu, J. (2010). Graphene/polyaniline nanofiber composites as supercapacitor electrodes. Chemistry of Materials, 22(4), 1392–1401
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mohseni, E., Rahmani, A. & Hamdi, Z. Poly (4,4′-methylenedianiline)-graphene oxide nanocomposite: synthesize and application in removal of benzothiophene from model liquid fuel. Environ Monit Assess 193, 737 (2021). https://doi.org/10.1007/s10661-021-09507-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-021-09507-y