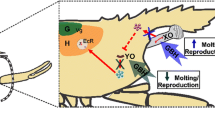
Abstract
Although medicines are less toxic than other toxicants, increased production and usage of pharmaceuticals have led to many concerns regarding their toxic effects on human and non-target organisms. Additionally, reproductive toxicity after long-term exposure is difficult to anticipate. Tamoxifen (TAM), a selective estrogen receptor modulator, has been widely used as an anticancer drug for mammalian breast and endometrial cancers. With increased TAM usage, it has frequently been reported that TAM is a potential endocrine disruptor capable of interfering with reproduction in non-target organisms. However, the mode of action of TAM in the endocrine system is unknown. In this study, we performed a 21-day chronic toxicity test using the crustacean Daphnia magna and investigated the transcriptional modulation of major genes related to the endocrine system, molting, development, and reproduction (i.e., Dm-vtg2, vmo1, cyp314, usp, and ecrb) after TAM exposure for 3, 6, 12, and 24 h. Our results showed a concentration-dependent decrease in the total number of offspring per individual, except for the concentration 25 μg/L; additionally, the expression of oogenesis-related genes was induced early but was later inhibited by TAM exposure. Additionally, molting-related genes were also downregulated in a time-dependent manner. Our findings suggested that TAM regulates reproduction by interfering with the molecular mechanisms involved in oogenesis and molting. This study supports the hypothesis that D. magna are a useful model to rapidly evaluate the reproductive effects of pharmaceuticals.

Similar content being viewed by others
References
Bolon, B., Bucci, T. J., Warbritton, A. R., Chen, J. J., Mattison, D. R., & Heindel, J. J. (1997). Differential follicle counts as a screen for chemically induced ovarian toxicity in mice: results from continuous breeding bioassays. Fundamental and Applied Toxicology: Official Journal of the Society of Toxicology, 39(1), 1–10.
Borgatta, M., Waridel, P., Decosterd, L. A., Buclin, T., & Chèvre, N. (2016). Multigenerational effects of the anticancer drug tamoxifen and its metabolite 4-hydroxy-tamoxifen on Daphnia pulex. The Science of the Total Environment, 545–546, 21–29.
Brown, M. R., Sieglaff, D. H., & Rees, H. H. (2009). Gonadal ecdysteroidogenesis in arthropoda: occurrence and regulation. Annual Review of Entomology, 54, 105–125.
Gilbert, L. I., Rybczynski, R., & Warren, J. T. (2002). Control and biochemical nature of the ecdysteroidogenic pathway. Annual Review of Entomology, 47, 883–916.
Gupta, R. C. (2018). Veterinary toxicology basic and clinical principles. Academic Press.
Hann, B. J. (1986). Revision of the genus Daphniopsis Sars, 1903 (Cladocera: Daphniidae) and a description of Daphniopsis chilensis, new species, from South America. Journal of Crustacean Biology, 6(2), 246–263.
Hannas, B. R., Wang, Y. H., Thomson, S., Kwon, G., Li, H., & Leblanc, G. A. (2011). Regulation and dysregulation of vitellogenin mRNA accumulation in daphnids (Daphnia magna). Aquatic Toxicology, 101(2), 351–357.
Heckmann, L. H., Connon, R., Hutchinson, T. H., Maund, S. J., Sibly, R. M., & Callaghan, A. (2006). Expression of target and reference genes in Daphnia magna exposed to ibuprofen. BMC Genomics, 7, 175.
Heckmann, L. H., Sibly, R. M., Connon, R., Hooper, H. L., Hutchinson, T. H., Maund, S. J., Hill, C. J., Bouetard, A., & Callaghan, A. (2008). Systems biology meets stress ecology: linking molecular and organismal stress responses in Daphnia magna. Genome Biology, 9(2), R40.
Houde, M., Douville, M., Gagnon, P., Sproull, J., & Cloutier, F. (2015). Exposure of Daphnia magna to trichloroethylene (TCE) and vinyl chloride (VC): evaluation of gene transcription, cellular activity, and life-history parameters. Ecotoxicology and Environmental Safety, 116, 10–18.
Hutchinson, T. H. (2002). Reproductive and developmental effects of endocrine disrupters in invertebrates: in vitro and in vivo approaches. Toxicology Letters, 131(1–2), 75–81.
Jagt, K. E. V. D., Munn, S., Torslov, J., & De Bruijn, J. (2004). Alternative approaches can reduce the use of test animals under REACH. EC Publishing PhysicsWeb http://publications.jrc.ec.europa.eu/repository/handle/JRC29111.
Jordan, V. C., Dix, C. J., Rowsby, L., & Prestwich, G. (1977). Studies on the mechanism of action of the nonsteroidal antioestrogen tamoxifen (ICI 46,474) in the rat. Molecular and Cellular Endocrinology, 7(2), 177–192.
Kato, Y., Kobayashi, K., Oda, S., Tatarazako, N., Watanabe, H., & Iguchi, T. (2007). Cloning and characterization of the ecdysone receptor and ultraspiracle protein from the water flea Daphnia magna. The Journal of Endocrinology, 193(1), 183–194.
Marie, P., Labas, V., Brionne, A., Harichaux, G., Hennequet-Antier, C., Rodriguez-Navarro, A. B., Nys, Y., & Gautron, J. (2015). Data set for the proteomic inventory and quantitative analysis of chicken eggshell matrix proteins during the primary events of eggshell mineralization and the active growth phase of calcification. Data in Brief, 7(4), 430–436.
Martin, M. T., Knudsen, T. B., Reif, D. M., Houck, K. A., Judson, R. S., Kavlock, R. J., & Dix, D. J. (2011). Predictive model of rat reproductive toxicity from ToxCast high throughput screening. Biology of Reproduction, 85(2), 327–339.
Muramatsu, M., & Inoue, S. (2000). Estrogen receptors: how do they control reproductive and nonreproductive functions? Biochemical and Biophysical Research Communications, 270(1), 1–10.
Namiki, T., Niwa, R., Sakudoh, T., Shirai, K., Takeuchi, H., & Kataoka, H. (2005). Cytochrome P450 CYP307A1/spook: a regulator for ecdysone synthesis in insects. Biochemical and Biophysical Research Communications, 337(1), 367–374.
Orias, F., Bony, S., Devaux, A., Durrieu, C., Aubrat, M., Hombert, T., Wigh, A., & Perrodin, Y. (2015). Tamoxifen ecotoxicity and resulting risks for aquatic ecosystems. Chemosphere, 128, 79–84.
Pagano, G., de Biase, A., Deeva, I. B., Degan, P., Doronin, Y. K., Iaccarino, M., Oral, R., Trieff, N. M., Warnau, M., & Korkina, L. G. (2001). The role of oxidative stress in developmental and reproductive toxicity of tamoxifen. Life Sciences, 68(15), 1735–1749.
Prot, J. M., & Leclerc, E. (2012). The current status of alternatives to animal testing and predictive toxicology methods using liver microfluidic biochips. Annals of Biomedical Engineering, 40(6), 1228–1243.
Rewitz, K. F., & Gilbert, L. I. (2008). Daphnia Halloween genes that encode cytochrome P450s mediating the synthesis of the arthropod molting hormone: evolutionary implications. BMC Evolutionary Biology, 25(8), 60.
Sannerstedt, R., Lundborg, P., Danielsson, B. R., Kihlström, I., Alván, G., Prame, B., & Ridley, E. (1996). Drugs during pregnancy. Drug Safety, 14(2), 69–77.
Seiler, A., Visan, A., Buesen, R., Genschow, E., & Spielmann, H. (2004). Improvement of an in vitro stem cell assay for developmental toxicity: the use of molecular endpoints in the embryonic stem cell test. Reproductive Toxicology, 18(2), 231–240.
Shibutani, S., Suzuki, N., Laxmi, Y. R. S., Schild, L. J., Divi, R. L., Grollman, A. P., & Poirier, M. C. (2003). Identification of Tamoxifen-DNA adducts in monkeys treated with Tamoxifen. Cancer Research, 63(15), 4402–4406.
Smirnov, N. N. (2014). The physiology of the Cladocera. Amsterdam: Academic Press.
US EPA. (2002). Methods for measuring the acute toxicity of effluents and receiving waters to freshwater and marine organisms. Cincinnati: Environmental monitoring systems laboratory.
Xia, L., Zheng, L., & Zhou, J. L. (2015). Transcriptional and morphological effects of tamoxifen on the early development of zebrafish (Danio rerio). Journal of Applied Toxicology, 36(6), 853–862.
Yang, G., Nowsheen, S., Aziz, K., & Georgakilas, A. G. (2013). Toxicity and adverse effects of Tamoxifen and other anti-estrogen drugs. Pharmacology & Therapeutics, 139(3), 392–404.
Funding
This study was funded by the Korea Ministry of Environment (MOE) as Technology Program for establishing biocide safety management (RE20184085) and a grant from the Korea Institute of Toxicology (KIT, Korea).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jo, M., Lee, S., Yoon, S. et al. Developmental and reproductive effects of tamoxifen on Daphnia magna. Environ Monit Assess 190, 677 (2018). https://doi.org/10.1007/s10661-018-7002-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-018-7002-y