Abstract
Hop cultivation, integral to the brewing industry, faces challenges from viroids, especially the citrus bark cracking viroid (CBCVd) but also the hop latent viroid (HLVd) influences hop cone quality. We focused on the degradation kinetics of HLVd thereby covering compost, silage, and digestate made from hop residues. In this study, HLVd serves as a model for understanding CBCVd, which causes significant stunting and yield losses in European hop crops. Composting experiments revealed that although composting significantly lowers HLVd levels, complete degradation within 7 weeks is not guaranteed, with loose compost showing a more rapid reduction than compacted variants. Infectivity experiments conducted using inocula obtained from HLVd-infected hop plant residues exposed to composting, ensiling, and biogas digestate did not result in the transmission of HLVd to viroid-free plants. Also extracting and analyzing the soil-root mixture of plants inoculated with HLVd-infected hop residues did not show evidence for viroid persistence. Degradation experiments further differentiated between the physiochemical and biological influences on viroid and viroid-like random RNA stability, showing that higher temperatures of 50 °C enhance degradation over 40 °C, and pH levels of 5 or 7 are slowing degradation. In contrast deionized water or a pH of 4 or 9 enhances viroid degradation. Adding extracts from digestate accelerated the process indicating a role of biological activity. Interestingly, a viroid-like random RNA with similar physiochemical properties, showed to degrade faster compared to HLVd, suggesting high robustness of the actual viroid secondary structure. These findings offer valuable insights into managing HLVd in hops and potentially other crops, highlighting effective strategies to mitigate viroid spread, and contributing to broader understanding of RNA degradation in agriculture.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Viroids, the smallest known infectious agents of plants, exert considerable pathogenic effects, including hop (Humulus lupulus L.). The cultivation of hops provides a key ingredient for the brewing industry. Hop cultivation is essential for both traditional alcoholic beers and the growing market for non-alcoholic beers (De Keukeleire, 2000; Hagemann et al., 2016). Hop is a known host for four viroids, the citrus bark cracking viroid (CBCVd, formerly CVd-IV), the hop stunt viroid (HSVd), the apple fruit crinkle viroid (AFCVd), and the hop latent viroid (HLVd). CBCVd, particularly devastating, often eludes early detection and can damage hop plants within a few years (Štajner et al., 2019). It was initially identified in Slovenia in 2007 and subsequently in Germany's Hallertau region in 2019, one of the world's leading hop-producing areas (Jakse et al., 2015; Julius Kühn-Institut, 2019). In Slovenia, about 300 hectares of hop fields were eradicated due to CBCVd (EPPO Global Database, 2021). In Germany 110 hectares were found to be CBCVd-infested by 2022 and are consequently under stringent monitoring (LfL, 2023). In 2023, the occurrence of CBCVd on hops was also reported from Brazil (Eiras et al., 2023). HSVd, known to cause stunted growth in hops, has been identified in a few instances in European hop cultivation (Radisek et al., 2012). However, it is more prevalent in the U.S., where it has been linked to stunting and yield losses in specific cultivars (Kappagantu et al., 2017). A hop-specific variant of AFCVd has so far only been found in hop plants in Japan (Sano et al., 2004). HLVd is globally distributed and considered to be present in all hop-producing countries (Puchta et al., 1988). While it causes only mild symptoms in some hop cultivars, HLVd has gained attention for its more pronounced stunting effects on cannabis plants (Adkar-Purushothama et al., 2023). However, close examinations of HLVd did reveal viroid-associated changes of hop cone quality, and potentially slightly reduced plant vigor and yield (Patzak et al., 2021).
Consequently, plant and plant residue hygiene emerge as a crucial element in hop production especially for avoiding viroid infections (Ling, 2017). Although the initial CBCVd infections might have originated from viroid-infected citrus fruits, which are easily to be found in supermarkets in hop growing regions (Hagemann et al., 2023), viroid spread within growing regions is predominantly through the use of infected plant materials, contaminated machinery, or residues from previous hop harvests (Radišek & Benko-Beloglavec, 2016). Specifically, hop production requires equipment including high trellises, pruning machinery, specialized harvesting trailers and machines, a kiln for drying, and further post-harvest processing pipelines. Therefore, here are several points where infections could be introduced or multiplied. In hop cultivation, the most significant byproducts are hop harvest residues, primarily composed of chopped bines from which most hop flowers have been removed (Görl et al., 2023). Görl et al. explain that these bines are often stored on farms before being reintroduced to hop gardens post-harvest in late autumn. The characteristics of these chopped bine piles, including initial high temperatures exceeding 60 °C and a phase of reduced oxygen levels, suggest a composting process (Görl et al., 2023). Although this composting serves as the principal method for managing hop harvest residues, the Hallertau region in Germany utilizes a biogas plant for this purpose. At this facility, hop residues undergo ensiling before serving as digester feedstock. Contrary to typical composting, silage involves an acidic anaerobic fermentation process, ideally at a pH of around four (Haag et al., 2015). However, due to its lower moisture content, hop silage ferments under less-than-ideal conditions, with pH values ranging from five to six, potentially accounting for the observed lack of viroid degradation in hop silage (Hagemann et al., 2021a, 2021b). This silage is then processed in the Hallertau biogas plant, where it is combined with maize or grass silage, resulting in the continuous release of fermentation byproducts, known as digestate. This digestate is ultimately returned to the hop fields. As a result, three primary hop byproducts are identified in the Hallertau region: compost, silage, and digestate, with compost and digestate being of practical importance as they are utilized as biofertilizers. Evaluating viroid degradation and the potential for post-treatment viroid infections in these byproducts is essential for risk assessment and the development of sanitation protocols for contaminated plant residues and equipment.
Viroids, especially those of the family Pospiviroidae, are remarkably stable due to high guanosine-cytosine-content, circularity, and extensive base pairing, creating a rod-like pseudo-helical structure (Riesner et al., 1983). They remain infective across various surfaces, except human skin, with sodium hypochlorite and dried skim milk as effective disinfectants (Mackie et al., 2015). HSVd, for instance, can infect plants even after exposure to 140 °C for 10 min (Takahashi & Yaguchi, 1985). However, measures which might be developed based on those methods do not seem to be feasible for hop harvest residue sanitation. Our research into ensiling hops showed that typical silage pH is too high for effective hygiene, with HLVd levels remaining constant over three months (Hagemann et al., 2021a, 2021b). In contrast, fermentation significantly reduced HLVd titer (Hagemann et al., 2021a, 2021b). Here 50 °C led to a significant drop in viroid titer after 5 days, whereas it took 30 days for at a fermentation temperature of 40 °C. This is in line with composting experiments conducted with potato spindle tuber viroid (PSTVd)-infected tomato residues (Kerins et al., 2018). Here higher temperatures reduce the time until viroid-infected tomato residues did not lead to reinfections after being composted for 7 days at 50 °C compared to 28 days at ambient temperature. These practical results align well with laboratory studies conducting temperature jump experiments with five different viroids; the results showed that a first clearly measurable structural transition occurs at 50 °C (Henco et al., 1977, 1979). It may be assumed that the viroids get more vulnerable to degradation at 50 °C than they are at colder temperatures.
Research on the physical properties of viroids and viroid degradation gets scarce after the mid-80s, but there is a growing body of research for exogenous (topically) applied double stranded RNAs (dsRNA) used in plant protection. In brief, dsRNAs get designed to disrupt the function of a pathogen or to trigger plant defense are sprayed onto plants (Rank & Koch, 2021). One study showed that dsRNA exposed to the environment can remain active for 5 but not for 20 days, if uncoated (Mitter et al., 2017). Other studies did show that dsRNA degrades within 4 or 7 days in water or in sediment–water systems, respectively (Albright et al., 2017; Fischer et al., 2017) or get adsorbed by soil particles or potentially degraded by microorganisms (Parker et al., 2019). These findings regarding environmentally exposed dsRNAs suggest that there is a strong biological component to RNA degradation. The separation of the purely physiochemical from the biological effects of RNases or other enzymes has not been studied for hop viroids to our knowledge. Here the fermentation experiments with HLVd-infected hop waste point to a potential source of highly active microbiota or enzymatic solutions to be tested for their contribution to viroid degradation.
This research into the biological and physiochemical degradation of RNAs is crucial for viroid research and other RNA-related disciplines, from agriculture and plant protection to RNA regulation and medicine. This study focuses on the (re-)infection potential of viroids in agriculture, presenting fundamental studies on viroid-like RNA degradation. Using HLVd as a model, we aim to fill knowledge gaps in viroid degradation, analyzing degradation during hop composting, seasonal variation in silage, the infection potential of hop residues, and to distinguish between physiochemical and biological conditions of viroid and viroid-like RNA degradation. The implications of this study are relevant for hop and hemp viroid management and help to develop strategies for the current CBCVd outbreak in European hop production and for mitigating the risk of HLVd for the cannabis industry. Beyond these practical considerations, the study contributes to broader understanding of RNA degradation and stability in agricultural contexts.
Materials and methods
Compost and ensiling experiment
Chopped HLVd-infected hop residues (bines and leaves) from cultivar 'Hallertauer Tradition' were aerobically composted in small-scale composters (1.2 m3 Big boxes, Auer packaging, Germany) at the Bavarian State Research Center for Agriculture (LfL) to simulate on-farm storage of hop harvest residues, as is common practice (Görl et al., 2021). For the compost preparation (800 kg per box), the compacted variant was achieved by evenly filling and manually stepping down the material in containers to ensure uniform density. The loose compost was filled and left uncompacted. Here natural aeration was possible due to the small pile size. Under practical conditions with larger piles, it is recommended to turn over the compost every 2 to 4 weeks (personal communication). Samples were taken before composting and after composting for 1, 2, 4, and 7 weeks. For each timepoint and condition, a fraction of the hop residue intended for sampling was filled into a rustling bag and placed in the center of an individual compost box. During the composting process temperature and oxygen levels (O2) were measured using a digital thermometer (TL253, Proster Trading Ltd., Hong Kong, China) and a portable gas analyzer (Dräger X-am 7000, Dräger Safety, Lübeck, Germany). Oxygen analysis consisted of an average of six measurements per composter. Both devices were mounted to 1.25 m long piercing probes to reach the center of the composter. After composting the samples were stored at -26 °C until further analysis.
Inoculation experiment
Inoculation with silage and digestate: The hop silage and digestate were taken from the Hallertau biogas plant, and positively tested for the presence of HLVd at the University of Hohenheim (UHOH), Stuttgart, Germany (Online Resource 1). Overall, the experiment was conducted at the Slovenian Institute of Hop Research and Brewing (IHPS), Žalec, Slovenia and at the UHOH. Vigorous, healthy, and viroid-free hop plants (Humulus lupulus L.) of approximately 30 cm height were used for the treatments. The experiments were conducted either with the cultivar `Celeia` at IHPS or with the cultivar `Saaz Osvald clone 31’ (hereafter referred to as `Saaz`) at the UHOH. The following was applied to 4 plants per treatment; (1) RNA extracted from digestate (2 µg/plant), (2) liquid extracted from digestate (100 µl/plant), (3) solid extract from digestate (50 g/plant) mixed with soil, (4) solid extract from hop silage (50 g/plant) mixed with soil, (5) non-infection control inoculation with water, and (6) HLVd-infection control inoculation with native RNA from HLVd-infected hop plants (2 µg/plant) extracted as described in the following. Additional treatments tested at the UHOH were (7) RNA extracted from hop silage (2 µg/plant), and (8) liquid extracted from hop silage (100 µl/plant). The inoculation were performed based on injecting RNA or liquid extract by using a syringe and needle as previously successfully established for inoculating hops with CBCVd (Gucek et al., 2019). The inoculation treatments with RNA or liquid extracts were conducted by stem injection of the inoculum twice at the 29.07.2021 and 18.08.2021 for the IHPS, and 12.07.2021 and 17.08.2021 at the UHOH. The solid silage incorporated into the soil of hop plants in the IHPS experiment did lead to the dieback of 3 out of 4 plants, assuming that the soil pH was lower due to the silage. However, at the UHOH experiment no phytotoxic effects were observed, which might be based on slight differences of the inoculation, which are that the silage residues were more equally distributed in the plan pots, while the silage was pressed towards the root in the IHPS experiment. For the UHOH inoculation experiment the RNA treatments may not have been successful as indicated by non-positive infection control plants. Therefore, a second RNA inoculation was performed successfully on the 13.10.2022 whereby the controls were infected as planned. Generally, plants of all treatments were grown in 1 L pots using commercial substrate. The hop plants were allowed to grow to a height of 1–1.5 m and then cut back. Biological pest control was carried out against spider mites. No other diseases or pests occurred. In addition, the plants were fully fertilized after the winter dormancy caused by annual total pruning in December. At the IHPS plants were maintained in growing chamber (Kambič RK-13300, Slovenia) under the following conditions: 16 h day at 25 °C and 60% relative humidity and 8 h night at 20 °C and 60%, respectively. At the UHOH plants were kept in a greenhouse under natural daylight conditions, while temperature was kept between 16–32 °C aided by air condition. At each sample date, newly formed leaves were collected from each plant and stored at -80 °C for further analysis.
RNA inoculum: For the preparation of viroid inoculum at the IHPS, RNA was extracted from 100 mg of plant tissue as described earlier (Kump & Javornik, 1996), and stored at -70 °C. The RNA concentration was measured using the Qubit® 3.0 Fluorometer (Thermo Fisher Scientific, USA). For the preparation of viroid inoculum at the UHOH RNA was extracted with the Monarch Total RNA Miniprep Kit (New England Biolabs, Ipswich, USA). Per sample 100 mg were homogenized by grinding using liquid nitrogen. Thereafter, RNA purity was determined with a spectrophotometer (Nanodrop 1000, Thermo Fisher, Waltham, USA). The samples were stored at -80 °C until further use.
Inoculation with compost: The stored samples from the compost experiment were stored at -30 °C until the infection experiment. Four viroid-free `Saaz` hop plants were soil inoculated per treatment at the UHOH. The treatments were a non-infection control containing 100 g substrate per plant. Further treatments were compacted or loose hop compost sampled after 1, 2, 4, or 7 weeks. For the treatments per plant 250 g of hop compost were introduced into the rhizosphere during repotting with 750 g substrate per plant on the 07.09.2022.
Viroid degradation experiment
To elucidate the degradation dynamics of viroids, we synthesized substantial quantities of HLVd-like molecules. A gene construct, termed 'copy machine' (CM), was created, comprising the T7 transcription start site followed by a single copy of the HLVd sequence variant (GenBank accession NC_003611). This construct was synthesized by Eurofins Genomics (GeneStrand service, Ebersberg, Germany) (see Online Resource 2 for sequence). PCR amplifications were conducted using the Q5 High Fidelity DNA Polymerase kit (New England Biolabs, Ipswich, USA), following a specific thermal cycling protocol: an initial step at 98 °C for 1 min, 35 cycles at 98 °C for 15 s, 56 °C for 30 s, and 72 °C for 30 s, concluding with a final extension at 72 °C for 3 min. The primers used for amplifying HLVd-DNA were T7-primer and HLVd_NC003611_R1 creating an amplicon spanning over the full length of the viroid (Online Resource 3). A 5 µl aliquot of the PCR product was evaluated through gel electrophoresis to confirm amplicon size, while the remaining volume (~ 40 µl) was purified using the Monarch PCR&DNA Cleanup Kit (New England Biolabs, Ipswich, USA).
Subsequently, RNA synthesis was carried out with the HiScribe T7 Quick High Yield RNA Synthesis Kit (New England Biolabs, Ipswich, USA) as per the standard protocol, including a 5-h incubation at 37 °C. Post-synthesis, the RNA was treated with DNase I (New England Biolabs, Ipswich, USA) to eliminate any residual DNA. The absence of DNA was confirmed via PCR, followed by RNA purification using the Monarch RNA Cleanup Kit (New England Biolabs, Ipswich, USA). The RNA was then ligated to acquire the viroid's natural conformation in vitro using the T4 RNA Ligase Kit (New England Biolabs, Ipswich, USA), adhering to the manufacturer's guidelines.
To assess the degradation of HLVd moleculaes in comparison to a synthetic viroid-like RNA, we designed an RNA molecule (RND) of the same length and guanosine-cytosine ratio as the HLVd sequence (256 nucleotides plus a 20 nucleotide T7 promoter; Online Resource 2). The RND sequence, synthesized similarly to HLVd NC_NC0036711, was random except for a specific 20-nucleotide segment (positions 142–162) targeted by the CVdIV_qPCR_Probe (Seigner et al. 2020), enabling RTqPCR quantification (Online Resource 3). For amplification, a combination of T7-primer and a newly designed primer, RND_VRDl_C_R1, along with its corresponding forward primer RND_VRDl_C_F1, were utilized in RTqPCR (Online Resource 4). The final RNA concentrations were confirmed through RTqPCR.
For the incubation experiment, we prepared a mixture containing 40 µl of either HLVd- or RND-molecule with 3,960 µl of buffer. This mixture was then transferred into a 15 ml Falcon tube. The incubation was conducted over periods of 0, 1, 3, 5, 15, and 30 days at two different temperatures, specifically 40 °C and 50 °C, in a dark environment to avoid any light-induced degradation or changes. The used buffers were a citric acid buffer at pH 4 (citric acid 10.76 g/l, sodium chlorite NaCl 2.56 g/l, sodium hydroxide NaOH 1 M 68 ml/l), a citric acid buffer at pH 5 (citric acid 18.52 g/l, sodium hydroxide NaOH 1 mol 196.4 ml/l), a potassium phosphate buffer at pH 7 (potassium dihydrogen phosphate KH2PO4 3.52 g/l, disodium phosphate Na2HPO4 * H20 7.26 g/l), Borax buffer at pH 9 (Borax Na2B4O7 * 10 H2O 4.78 g/l, hydrogen chloride 46 ml/l), and unbuffered double deionized water at pH 7. Per sample occasion 10 µl were used for the RTqPCR analysis. In addition to the pure chemical and temperature effects, a crude extract from the digestate was produced by grinding it in liquid nitrogen and then incubating 1 g mixed with 9 ml of each buffer for 24 h at 39 °C under agitation. Next the solutions were centrifuged at 5,000 g for 30 min and supernatant was used for mixing with the viroid- or RND-molecule after readjusting the pH with either HCl or NaOH.
RNA extraction and purification
In the viroid screening of silage and digestate from the Hallertau biogas plant (Online Resource 1), plant material from the compost experiment as well as from the UHOH inoculation experiment, the following methodology was applied: Frozen samples were ground using liquid nitrogen, with approximately 200 mg of the material used for RNA extraction. This extraction utilized the Monarch Total RNA Miniprep Kit (New England Biolabs, Ipswich, USA), following the protocol previously detailed (Hagemann et al., 2021a, 2021b). The RNA concentration and purity were determined by spectrophotometry, analyzing 2 µl of each sample with a Nanodrop 1000 (Thermo Fisher, Waltham, USA). For the IHPS segment of the inoculation experiment, RNA extraction started with 100 mg of plant tissue using the method described in Kump and Javornik (1996), with the concentration measured using a Qubit® 3.0 Fluorometer (Thermo Fisher Scientific, USA), and stored at -70 °C. It was observed that the RNA samples from the compost experiment exhibited lower purity. To address this, further purification was conducted using the Monarch RNA Cleanup Kit (New England Biolabs, Ipswich, USA), following the manufacturer’s guidelines.
For alternative RNA extraction involving roots and soil from inoculated plants, the following steps were conducted: roots were washed, freeze-dried, and then stored at 2 °C. The roots were ground in liquid nitrogen, and approximately 1 g was used for RNA extraction with the Qiagen RNeasy PowerSoil Total RNA Kit (Qiagen, Hilden, Germany). This extraction was performed according to the manufacturer's instructions, and the resultant RNA pellet was dissolved in 25 µl of elution buffer. The concentration and purity of these RNA samples were assessed using 2 µl on the spectrometer. RNA was stored at -80 °C until RTqPCR analysis.
PCR analysis
For the inoculation experiment involving silage, digestate, and compost, RNA samples were assessed using one-step reverse transcriptase PCR (RT-PCR) (OneStep RT-PCR Kit, Qiagen, Hilden, Germany). The primers for HLVd detection were HLVd-P and HLVd-M (Online Resource 4). Amplicons were initially evaluated via agarose gel electrophoresis, based on migration size, and exemplary amplicons were further validated through Sanger sequencing (Eurofins Genomics, Ebersberg, Germany). For the RTqPCR analysis RNA extracts were prepared in 1 µl volumes and tested in dilutions of 1:5 and 1:20 to address potential inhibitory effects. During analyzing the samples from the composting experiment, the nad5 internal control was found to be inconsistent in hop compost samples, however, nad5 showed to be more robust than the also tested mitochondrion cytochrome oxidase subunit I (CyCOX) (Online Resource 4). The actual target HLVd was detectable in 96 out of 128 runs, thus the internal positive control was not critical for the experimental analysis in the context of this degradation study, but this RTqPCR reaction should not be used for HLVd diagnostical purposes. Furthermore, the previously established internal PCR control for detecting 16S-RNA of archaea worked well for the digestate samples (Hagemann et al., 2021a, 2021b).
Statistical analysis
In our study, we conducted a detailed statistical analysis of the infection experiments and the degradation study. The infection experiment results are presented as counts, with the degradation study averages presented for clarity. To enhance readability, all values above 32 in the degradation study were set to 32, which is the threshold for reliable HLVd detection (Hagemann et al., 2021a, 2021b). This decision was based on the rationale that higher values tend to represent the absence of the viroid and are less informative for statistical analysis. We performed all analyses using R 4.3.2 (R Core Team, 2016) and RStudio 2023.03.0.
In our RTqPCR analysis of both the above-ground plant material and the root-soil extract from compost and other inoculations with solid material, we addressed missing data points by assigning a default Cq-value of 36. This value was chosen to distinctly represent the absence of detectable viroids, as opposed to a Cq-value of 32, which we had previously used to indicate a lower certainty region. This distinction is important as a Cq-value of 36 unequivocally signifies the absence of data due to low or no target (HLVd). This approach allowed for quantitative statistical analysis in the face of incomplete data. For the analysis, we employed a linear model to examine the effects of treatment, week, and dilution. Recognizing that dilution was a technical consideration rather than a biological variable, we averaged the results across dilution levels to emphasize the biological relevance of our findings.
Regarding the compost temperature data, we first extracted the weekly maximum temperatures. We then applied linear models to both temperature and oxygen level data to ascertain the impact of treatment and time. The significance of differences between groups in all statistical analyses was determined using the Tukey method for multiple comparison tests, maintaining a significance level (alpha) of 0.05.
Results
Compost experiment
We observed a significant reduction in HLVd levels after one week of composting when compared to fresh bines (Table 1). Interestingly, HLVd levels did not consistently decline over time, despite treatment and duration showing significant effects. The method of storing compost—either loosely or by compacting hop bines—significantly influenced the rate of viroid degradation. Specifically, loosely stored compost exhibited high Cq-values (> 35) after one week, suggesting very low viroid titers. In contrast, compacted forms showed mean Cq-values around 30, indicating reduced yet reliably detectable viroid levels. Sanger sequencing of select amplicons confirmed HLVd presence in 7 of 8 samples, with Cq-values ranging from 25 to 35. The effectiveness of loosely stored hops in composting raises questions about the underlying biological processes. Key composting parameters, such as temperature and oxygen content, were assessed (Table 1, Online Resource 5). The hop composting in this study started with a thermophilic phase in the first week and then gradually declined towards ambient conditions. Specifically, the temperatures peaked at 71.8 °C for loosely stored compost and reached 59.6 °C for compacted compost (Table 1, Online Resource 5). Oxygen levels started low (6–16%) and oxygen levels of loosely stored compost increased to more than 10% within two weeks, while compacted compost showed significantly lower oxygen levels below 10% for four weeks (Online Resource 6). Further analysis revealed a strong correlation between RTqPCR results and maximum temperature, with an R2 of 0.77, indicating a significant relationship between temperature and viroid degradation rates. Additionally, correlating RTqPCR results with oxygen measurements yielded an R2 of 0.54.
Inoculation with viroid-infected hop residues
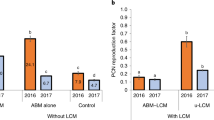
As previously mentioned, HLVd was detectable in compost samples. Over 2.5 years, HLVd consistently appeared at high titers in silage samples from the biogas plant (Online Resource 1) and was also verified before infection. Previous research indicated HLVd's presence in digestate (Hagemann et al., 2021a, 2021b). For our study, we prepared four plants per treatment with three different inocula types: solid, liquid, and RNA. Conducting experiments at two independent laboratories, UHOH and IHPS, enhanced the robustness of our findings (Fig. 1a and b). Notably, none of the 40 plants treated with HLVd-infected hop material showed infection (Fig. 1a and b). At UHOH, the initial infection attempt failed, necessitating reinfection (Fig. 1a). However, the infection was successful on the first attempt at the IHPS (Fig. 1b). As demonstrated (Table 1), compacted hop residue notably contained higher viroid titers (lower Cq-levels), and Sanger sequencing confirmed HLVd in the inoculum. However, using either loose or compacted hop compost as inoculum did not result in infections among 32 viroid-free plants (Fig. 1c). In total, 72 susceptible hop plants were inoculated with HLVd-infected hop matrices without a single case of infection, while control infections were finally successfully established in all experiments.
Comparative analysis of Hop Latent Viroid (HLVd) infection across different treatments, utilizing four plants per treatment, inoculated either via syringe (syringe icon) for liquid and RNA extracts, or mixed into the soil (shovel icon). Post-winter dormancy, the HLVd-infection status was evaluated by RT-PCR. (A) At the University of Hohenheim (UHOH), Germany, solid, liquid and RNA of digestate and silage were inoculated into viroid-free ‘Saaz’ hop plants; reinoculation was required due to control issues (red arrow). (B) At the Slovenian Institute of Hop Research and Brewing (IHPS), solid, liquid and RNA of digestate and solid silage were applied to ‘Celeia’ hop plants, with a pre-dormancy test included. (C) In the Bavarian State Research Center for Agriculture (LfL), Germany, compost from HLVd-infected hop harvest residues was produced at different durations (1, 2, 4, 7 weeks) for testing its impact on HLVd levels in loose and compacted forms. The compost was later inoculated into viroid-free ‘Saaz’ hop plants and tested at the UHOH
Apart from testing the above-ground plant for HLVd-infections we also examined the root substrate mixtures with the more sensitive RTqPCR. This expansion was critical for evaluating the presence and degradation of HLVd in a more inclusive and thorough manner. A first finding from this analysis concerned the RTqPCR; the effect of dilution levels on HLVd detection, as presented in Table 2 showed to be significant, unlike the results obtained from above-ground plant samples. Higher dilution of samples corresponded to increased Cq-values, and in some instances, there was no Cq-value at all, consequently we suggest using only undiluted soil extracts for soil viroid testing.
The Cq-value for the HLVd-infection control stood at a Cq-value of 23.5 on average (Table 2). Four out of four HLVd-infection control plants showed HLVd through low Cq-values and validated through Sanger sequencing of the corresponding RTqPCR amplicons. This observation is in congruence with the results obtained from above-ground plant material (Fig. 1), reinforcing that the soil root extraction method employed in this study effectively identifies viroids in hop root soil samples. In contrast, the treatments involving digestate solids and silage solids yielded significantly higher Cq-values, at 34.5 and 35.2 respectively. These values are above the reliable detection threshold previously reported as a Cq-value of 32 (Hagemann et al., 2021a, 2021b), hinting at either a diminished presence or potential absence of HLVd. Similarly, the negative control samples showed a Cq-value of 35.3, closely mirroring the outcomes of digestate and silage treatments. Moreover, our investigation into the temporal effects of compost inoculations revealed no significance depending on the number of weeks of composting for either of the treatments—compacted or loose. For the compost samples with Cq-values exceeding the reliable detection threshold of 32, six PCR products were randomly selected for sequencing to further investigate the presence of HLVd. This analysis revealed that four of these six samples were indeed positive for HLVd, indicating that despite low viroid titers suggested by high Cq-values, HLVd was still detectable in a subset of samples (Table 2).
Degradation experiment
In this experiment we evaluated the impact of pH, temperature, and the presence of biological material on HLVd degradation over a 30-day period under controlled conditions (Fig. 2). The degradation of HLVd was less effective at 40 °C compared to 50 °C, particularly at a pH of 9 and in deionized water (Fig. 2a and b). Moderate pH levels of 5 and 7 in contrast seem to preserve HLVd at both temperatures (Fig. 2a and b). The addition of an extract made from hop digestate did facilitate viroid degradation (Fig. 2c and d). Here, extract at a pH of 7 and 9 showed the best degradation effect against the viroid. Interestingly, deionized water treatments are among the most efficient at promoting degradation overall, leading to a substantial reduction in viroid levels across both temperature settings after 15 days (Fig. 2a-f). However, high acidity (pH 4) consistently facilitates the most effective degradation across all conditions. Further, HLVd was compared to a random synthetic RNA. The degradation patterns of the synthetic random RNA sequences are similar as seen for HLVd (Fig. 2a and b). At the higher temperature of 50 °C, the reduction in RNA concentration is rapid. Additionally, there was a diminished stability observed for the random RNA sequence when compared to HLVd at the same conditions, i.e. high HLVd titer at 40 °C and pH 9 at day 30, while low titer of the random RNA sequence (Fig. 2a and e). Further there was a higher HLVd titer at 50 °C and pH 9 at day 5 and 15 compared to a lower titer of the random RNA sequence at the corresponding days, respectively (Fig. 2b and f).
Degradation of hop latent viroid (HLVd) compared to a synthetic random RNA viroid. The heatmap of the degradation over a 30-day period of the hop latent viroid (HLVd, A-D) compared to a synthetic random RNA viroid (Random, E, F). The heatmap displays degradation patterns of the two RNAs at temperatures of 40 °C (A, C, E) and 50 °C (B, D, F), evaluated through RTqPCR (Cq-value) at 0, 1, 3, 5, 15, and 30 days. The color gradient represents the Cq-value: dark red low values corresponding to high viroid titers, transitioning to dark blue for high Cq values, which equals the detection threshold. Rows indicate varying pH levels (4, 5, 7, 9) and the deionized water (ddH2O)
Discussion
Compost experiment
Extending the approach of managing viroid-infected plant residues through composting, as demonstrated with PSTVd-infected tomato plants by Kerins et al. (2018), this study delves into the degradation of HLVd-infected hop residues. Overall, the composting process indeed led to reduced HLVd levels when comparing the RTqPCR values for any given composting treatment to fresh bines (Table 1). However, HLVd levels were not constantly declining over time as expected, though treatment and time showed a highly significant effect. Storing compost loosely versus compacting hop bines before on-farm storage affected viroid degradation rate; the loose variant had high Cq-values of 36 on average, indicating a very low viroid titer at all time points, while the compacted form had a mean Cq-value of 30, indicating reduced but still reliably measurable viroid levels (Table 1).
Generally, the composting as conducted in this study followed typical properties of (hop) composting, i.e. a thermophilic phase of high temperatures above 60 °C along with low oxygen levels within the composting pile (Görl et al., 2023). These dynamic changes in temperature and oxygen levels are primarily driven by the intense metabolic activity of microorganisms during the early composting process. The initial high temperatures result from rapid microbial decomposition, while the subsequent increase in oxygen levels reflects the slowing down of microbial activity as the compost matures (Azim et al., 2018). Our measurements align with this notion, moreover the treatments, loosely stored versus compacted, did lead to highly significant differences regarding temperature and oxygen, whereas the peak temperature of the loosely stored compost was 12 °C higher compared to compacted compost (Online Resource 5). This higher temperature might explain the lower viroid titer found in the loosely stored compost and is in line with earlier findings that HLVd degradation is a temperature-driven process (Hagemann et al., 2021a, 2021b). Further, examining through correlation analysis support the importance of the temperature effect; a notable higher correlation was found between RTqPCR results and maximum temperature (R2 of 0.77) when compared to a lower correlation for oxygen (R2 of 0.54), indicating that peak temperatures is the pivotal factors in HLVd degradation.
Further, the oxygen levels of loosely stored compost quickly went back to above 10% within 2 weeks, while it remained lower than 10% in the first 4 weeks in compacted compost, which is indicative of an anaerobic environment (Online Resource 4), similar to ensiling, which has been shown to preserve HLVd over months (Hagemann et al., 2021a, 2021b). This underscores the potential benefit of loosely storing hop harvest residues as a measure to enhance viroid degradation through composting, although this method may not guarantee complete elimination of HLVd.
Given the physiochemical similarities between HLVd and other viroids that infect hops, like CBCVd, our findings suggest that the strategies effective for HLVd may also hold promise for managing CBCVd. This study, therefore, not only highlights the potential of composting as a viable strategy for reducing viroid levels in hop production but also calls for further research to optimize composting conditions for effective pathogen management.
Inoculation with viroid-infected hop residues
Building on our understanding of HLVd persistence in hop-related substrates, this study, alongside prior research (Hagemann et al., 2021a, 2021b), underscores the resilience of HLVd in various contexts. Building on the previous study we now investigate the infection potential of silage and digestate by using HLVd-infected materials directly sourced from the Hallertau biogas plant instead of laboratory made material. This emphasizes the practical relevance of our infectivity assessments for comprehensive viroid risk analysis. Our findings consistently indicated the absence of HLVd transmission to hop plants, regardless of the inoculum form used (Fig. 1). Overall, we tested 40 plants in May or later, which has shown to be crucial to avoid overlooking infections (Morton et al., 1993). Morton et al. (1993) found out that HLVd survives in the rootstock. We assumed that after viroid infection, the viroids may establish locally, then get translocated into the rootstock in the dormancy phase from where they gradually spread throughout most of the plant until May as shown by Morton et al. (1993). Further, Ziegler et al. (2014) also found HLVd to be more abundant in mature leaves and later in the season. This is in line with our findings of only two out of four HLVd-infection controls being positive in June 2023, but four out of four in August 2023 (Fig. 1). This highlights that multiple testing is recommendable for viroids studies and further that tests should be performed during the summer months to ensure accurate detection of infections (Morton et al., 1993; Ziegler et al., 2014).
However, in our study we did add another level of testing, which is the below ground testing with RTqPCR for HLVd in the root soil mixture. The results backed up the data for the above ground RT-PCR for silage solid and digestate solid treatments as well as for the HLVd-infection control. Further, the plants treated with eight treatments, two types of hop compost stored for four durations also did not show any infections (Fig. 1c) despite the RTqPCR did indicate high viroid titers in compacted compost (Table 1). Accumulating our results shows that across 72 HLVd-susceptible hop plants treated with HLVd-infected hop material none yielded in HLVd-infections. Further, our exploration into root substrate mixtures with RTqPCR analysis may be used for off-season viroid detection in rootstock during winter periods when no above ground plant material is available. This would also support the distribution of viroid-free plants for planting needed for sustainable plant trade and extending production areas.
Degradation experiment
In our previous study on HLVd we investigated HLVd degradation at temperatures possible during the biogas fermentation process, 40 °C and 50 °C. We found out that the degradation was accelerated at 50 °C than at 40 °C until the viroid was significantly reduced (Hagemann et al., 2021a, 2021b). This study does continue to investigate the impact of pH, temperature, and the presence of biological material on HLVd degradation over a 30-day period under controlled conditions (Fig. 2). We could verify our data by showing again that also under these conditions the degradation of HLVd was enhanced by 50 °C compared to 40 °C, particularly at a pH of 4 and in deionized water (Fig. 2a and b). The effect of deionized water may be explained by an atomic force microscopy study on the native structure of PSTVd showing that manganese is important for stabilizing the rod-like shape of this viroid (Moreno et al., 2019); we hypothesize that in the absence of ions in deionized water the viroid is destabilized, thus making it more vulnerable to degradation. However, using deionized water may not have a direct use under practical conditions but for diagnostics we recommend to used buffered solutions for viroid handling and storage.
The effect of deionized water as well as all other pH values and treatments were enhanced by 50 °C without exception (Fig. 2). This finding supports the crucial role of temperature in hastening the degradation process. This is also in line with temperature jump studies of other viroids, which show structural change at about 50 °C (Henco et al., 1977, 1979), probably related to less base pairings, larger bulbs, thus better access for physiochemical degradation or RNases. Modeling the secondary structure of HLVd and CBCVd also supports this notion, showing that more nucleotides get unpaired and the organizational level decreases as temperature increases until the secondary structure is lost above 70 °C (Hagemann et al., 2021a, 2021b). The degradation at pH 9 in the absence of enzymatic activity was suggested to be a purely physiochemical reaction of RNA degradation based on the cleavage and transesterification of the phosphor backbone of the RNA nucleotide enhanced by increasing the pH-value and higher temperatures (Li & Breaker, 1999). Moderate pH levels of 5 and 7 in contrast seem to preserve HLVd at both temperatures (Fig. 2a and b). Experiments with disinfecting tools from the tomato chlorotic dwarf viroid with crude sap of pepper plants show that biological matrices can have an effect close to harsh chemicals such as sodium hypochlorite (Matsuura et al., 2010). In our study, we tested a matrix easily available, extract from digestate, and indeed it appears to facilitate viroid degradation, potentially emulating fermenter environment effects (Fig. 2c and d). Here, a pH of 7 and 9 seems optimal for enzyme activity within the extract since this treatment showed a viroid reduction after one day already. Overall, high acidity (pH 4) consistently facilitates the most effective degradation across all conditions.
The degradation patterns of synthetic random RNA sequences are overall similar, except for the diminished stability compared to HLVd (Fig. 2). This finding might imply that the natural viroid is more resilience compared to the synthetic RNA sequence, despite both entities sharing similar properties, such as forming a rod-like structure, having an identical guanosine-cytosine ratio, and being of the same length. These observations suggest fundamental differences in their degradation behaviors, underscoring inherent disparities between the actual viroid and its synthetic counterpart. This finding is in line with another study including random sequences with viroid-like properties, where it was shown through melting curve that viroid stability is associated with the distinct secondary structures of viroids, rather than simply with high GC-content (Sieger et al., 1984).
Conclusion
Our study underscores the significant role of processing and environmental conditions in the degradation of viroids, particularly HLVd, within agricultural systems. The persistence of HLVd in on-farm hop compost or biogas plant silage and digestate is a challenge for hop growers or biogas plant operators, respectively, emphasizing the need for effective management strategies to mitigate the risk of (re-)infection. The findings from our degradation experiments offer promising insights; specifically, they reveal that higher temperatures and acidic pH 4 conditions can considerably expedite the degradation process, potentially reducing the reinfection potential of viroid-contaminated residues when reintroduced as green manure. Our research provides evidence that composting under controlled conditions, especially with adequate aeration and moisture, can significantly lower HLVd titers, bringing them close to or below detection limits. This insight is crucial for hop growers who traditionally rely on the reuse of hop residues as organic fertilizer. By adopting composting practices that increase temperatures to 50 °C or more, such as frequent turning and proper pile management, the risk of spreading viroids through compost can be minimized.
Furthermore, our inoculation experiments, which did not result in viroid transmission to viroid-free plants, suggest that the infectious potential of HLVd in hop residues is low. However, the absence of infection in our trials does not prove the absence of a residual risk for infection, especially in field conditions. Therefore, continuous monitoring and precautionary measures remain essential.
Our results are in line with results conducted with PSTVd composting and results from practical experiments match laboratory trials and related literature for viroids. Therefore, we would assume that the overall results for HLVd can be principally used as starting point for development of practical solutions for current CBCVd outbreaks in European hops, but also for HLVd management in the hemp and cannabis industry, or generally for managing viroids in horticultural practices. The research further contributes to a broader understanding of RNA degradation in the environment. This knowledge can inform future viroid or even dsRNA management practices and biosecurity measures across multiple cropping systems, ensuring the sustainability and productivity of crucial agricultural industries.
Data availability
Datasets and protocols are available from the corresponding author.
References
Adkar-Purushothama, C. R., Sano, T., & Perreault, J.-P. (2023). Hop latent viroid: A hidden threat to the cannabis industry. Viruses, 15(3), 681. https://doi.org/10.3390/v15030681
Albright, V. C., Wong, C. R., Hellmich, R. L., & Coats, J. R. (2017). Dissipation of double-stranded RNA in aquatic microcosms. Environmental Toxicology and Chemistry, 36(5), 1249–1253. https://doi.org/10.1002/etc.3648
Azim, K., Soudi, B., Boukhari, S., Perissol, C., Roussos, S., & Thami Alami, I. (2018). Composting parameters and compost quality: A literature review. Organic Agriculture, 8(2), 141–158. https://doi.org/10.1007/s13165-017-0180-z
De Keukeleire, D. (2000). Fundamentals of beer and hop chemistry. Química Nova, 23(1), 108–112. https://doi.org/10.1590/S0100-40422000000100019
Eiras, M., de Oliveira, A. M., de Fátima Ramos, A., Harakava, R., & Daròs, J.-A. (2023). First report of citrus bark cracking viroid and hop latent viroid infecting hop in commercial yards in Brazil. Journal of Plant Pathology, 105(2), 603–603. https://doi.org/10.1007/s42161-023-01313-4
EPPO Global Database. (2021). Citrus bark cracking viroid. EPPO Datasheet. https://gd.eppo.int/taxon/CBCVD0/datasheet. Assessed 29 Apr 2024.
Fischer, J. R., Zapata, F., Dubelman, S., Mueller, G. M., Uffman, J. P., Jiang, C., et al. (2017). Aquatic fate of a double-stranded RNA in a sediment–water system following an over-water application. Environmental Toxicology and Chemistry, 36(3), 727–734. https://doi.org/10.1002/etc.3585
Görl, J., Lohr, D., & Meinken, E. (2021). Nitrogen release from aerobically composted hop bines. Acta Horticulturae, 1328(1328), 121–126. https://doi.org/10.17660/ActaHortic.2021.1328.17
Görl, J., Lohr, D., Meinken, E., & Hülsbergen, K.-J. (2023). Co-composting of hop bines and wood-based biochar: Effects on composting and plant growth in copper-contaminated soils. Agronomy, 13(12), 3065. https://doi.org/10.3390/agronomy13123065
Gucek, T., Jakše, J., & Radisek, S. (2019). Testing of different methods for mechanical inoculation of plants with CBCVd. Hop Bulletin, 26, 36–50. https://www.researchgate.net/publication/338864137_Preizkusanje_razlicnih_metod_okuzevanja_rastlin_s_CBCVdTesting_of_different_methods_for_mechanical_inoculation_of_plants_with_CBCVd. Accessed 6 April 2020
Haag, N. L., Nägele, H. J., Fritz, T., & Oechsner, H. (2015). Effects of ensiling treatments on lactic acid production and XXXXXXXXlementary methane formation of maize and amaranth. Bioresource Technology, 178, 217–225. https://doi.org/10.1016/j.biortech.2014.08.048
Hagemann, M. H., Born, U., Sprich, E., Seigner, L., Oechsner, H., Hülsemann, B., et al. (2021b). Degradation of hop latent viroid during anaerobic digestion of infected hop harvest residues. European Journal of Plant Pathology, 161(3), 579–591. https://doi.org/10.1007/s10658-021-02344-2
Hagemann, M. H., Treiber, C., Born, U., Schrader, G., Stampfl, J., Jakše, J., & Radišek, S. (2023). Risk potential of international fruit trade for viroid spreading - case study on hop viroids in Europe. Journal of Plant Pathology, 1(1), 1. https://doi.org/10.1007/s42161-023-01449-3
Hagemann, M. H., Bogner, K., Marchioni, E., Braun, S., & Susanne, B. (2016). Chances for dry-hopped non-alcoholic beverages? Part 1: Concept and market prospects. BrewingScience, 69(7–8), 50–55. http://www.brewingscience.de/index.php?tpl=table_of_contents&year=2016&edition=0007%2F0008&article=88401. Accessed 29 Apr 2024.
Hagemann, M. H., Born, U., Sprich, E., Seigner, L., Oechsner, H., Hülsemann, B., et al. (2021). Fate of the hop latent viroid during ensiling of hop harvest residues. Acta Horticulturae, (1328), 67–74. https://doi.org/10.17660/ActaHortic.2021.1328.9
Henco, K., Riesner, D., & Sanger, H. L. (1977). Conformation of viroids. Nucleic Acids Research, 4(1), 177–194. https://doi.org/10.1093/nar/4.1.177
Henco, K., Sänger, H. L., & Riesner, D. (1979). Fine structure melting of viroids as studied by kinetic methods. Nucleic Acids Research, 6(9), 3041–3059. https://doi.org/10.1093/nar/6.9.3041
Jakse, J., Radisek, S., Pokorn, T., Matousek, J., & Javornik, B. (2015). Deep-sequencing revealed citrus bark cracking viroid (CBCVd) as a highly aggressive pathogen on hop. Plant Pathology, 64(4), 831–842. https://doi.org/10.1111/ppa.12325
Julius Kühn-Institut. (2019). First finding of citrus bark cracking viroid (CBCVd) in Germany (Bavaria). Braunschweig, Germany. https://pflanzengesundheit.julius-kuehn.de/dokumente/upload/CBCVd_pr2019-08by.pdf. Accessed 4 January 2022
Kappagantu, M., Villamor, D. E. V., Bullock, J. M., & Eastwell, K. C. (2017). A rapid isothermal assay for the detection of hop stunt viroid in hop plants (Humulus lupulus), and its application in disease surveys. Journal of Virologic Methods, 245(August 2016), 81–85. https://doi.org/10.1016/j.jviromet.2017.04.002
Kerins, G., Blackburn, J., Nixon, T., Daly, M., Conyers, C., Pietravalle, S., et al. (2018). Composting to sanitize plant-based waste infected with organisms of plant health importance. Plant Pathology, 67(2), 411–417. https://doi.org/10.1111/ppa.12729
Kump, B., & Javornik, B. (1996). Evaluation of genetic variability among common buckwheat (Fagopyrum esculentum Moench) populations by RAPD markers. Plant Science, 114(2), 149–158. https://doi.org/10.1016/0168-9452(95)04321-7
LfL. (2023). Jahresbericht 2022 Sonderkultur Hopfen. Wolnzach, Germany. https://www.lfl.bayern.de/mam/cms07/publikationen/daten/informationen/jahresbericht-hopfen-2022_lfl-information.pdf. Accessed 29 Apr 2024.
Li, Y., & Breaker, R. R. (1999). Kinetics of RNA degradation by specific base catalysis of transesterification involving the 2γ-hydroxyl group. Journal of the American Chemical Society, 121(23), 5364–5372. https://doi.org/10.1021/ja990592p
Ling, K.-S. S. (2017). Decontamination measures to prevent mechanical transmission of viroids. In Viroids and Satellites pp 437–445. Elsevier Inc. https://doi.org/10.1016/B978-0-12-801498-1.00041-3
Mackie, A. E., Coutts, B. A., Barbetti, M. J., Rodoni, B. C., McKirdy, S. J., & Jones, R. A. C. (2015). Potato spindle tuber viroid: Stability on common surfaces and inactivation with disinfectants. Plant Disease, 99(6), 770–775. https://doi.org/10.1094/PDIS-09-14-0929-RE
Matsuura, S., Matsushita, Y., Usugi, T., & Tsuda, S. (2010). Disinfection of tomato chlorotic dwarf viroid by chemical and biological agents. Crop Protection, 29(10), 1157–1161. https://doi.org/10.1016/j.cropro.2010.05.018
Mitter, N., Worrall, E. A., Robinson, K. E., Li, P., Jain, R. G., Taochy, C., et al. (2017). Clay nanosheets for topical delivery of RNAi for sustained protection against plant viruses. Nature Plants, 3(January), 16207. https://doi.org/10.1038/nplants.2016.207
Moreno, M., Vázquez, L., López-Carrasco, A., Martín-Gago, J. A., Flores, R., & Briones, C. (2019). Direct visualization of the native structure of viroid RNAs at single-molecule resolution by atomic force microscopy. RNA Biology, 16(3), 295–308. https://doi.org/10.1080/15476286.2019.1572436
Morton, A., Barbara, D. J., & Adams, A. N. (1993). The distribution of hop latent viroid within plants of Humulus lupulus and attempts to obtain viroid-free plants. Annals of Applied Biology, 123(1), 47–53. https://doi.org/10.1111/j.1744-7348.1993.tb04071.x
Parker, K. M., Barragán Borrero, V., Van Leeuwen, D. M., Lever, M. A., Mateescu, B., & Sander, M. (2019). Environmental fate of RNA interference pesticides: Adsorption and degradation of double-stranded RNA molecules in agricultural soils. Environmental Science and Technology, 53(6), 3027–3036. https://doi.org/10.1021/acs.est.8b05576
Patzak, J., Henychová, A., Krofta, K., Svoboda, P., & Malířová, I. (2021). The influence of hop latent viroid (HLVd) infection on gene expression and secondary metabolite contents in hop (Humulus lupulus L.) Glandular Trichomes. Plants, 10(11), 2297. https://doi.org/10.3390/plants10112297
Puchta, H., Ramm, K., & Sänger, H. L. (1988). The molecular structure of hop latent viroid (HLV), a new viroid occurring worldwide in hops. Nucleic Acids Research, 16(10), 4197–4216. https://doi.org/10.1093/nar/16.10.4197
R Core Team. (2016). R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2014. Vienna, Austria. https://www.r-project.org/. Accessed 29 Apr 2024.
Radisek, S., Majer, A., Jakse, J., Javornik, B., & Matoušek, J. (2012). First report of hop stunt viroid infecting hop in slovenia. Plant Disease, 96(4), 592–592. https://doi.org/10.1094/PDIS-08-11-0640-PDN
Radišek, S., & Benko-Beloglavec, A. (2016). Pest risk analysis for citrus bark cracking viroid (CBCVd). Slovenian Institute of Hop Research and Brewing. Administration of the Republic of Slovenia for Food Safety, Veterinary Sector and Plant Protection. European Plant Protection Organization. https://gd.eppo.int/taxon/CBCVD0/documents. Accessed 29 Apr 2024.
Rank, A. P., & Koch, A. (2021). Lab-to-field transition of rna spray applications – how far are we? Frontier in Plant Science, 2, 2243. https://doi.org/10.3389/FPLS.2021.755203
Riesner, D., Steger, G., Schumacher, J., Gross, H. J., Randles, J. W., & Sänger, H. L. (1983). Structure and function of viroids. Biophys of Structure and Mechanism, 9(3), 145–170. https://doi.org/10.1007/BF00537813
Sano, T., Yoshida, H., Goshono, M., Monma, T., Kawasaki, H., & Ishizaki, K. (2004). Characterization of a new viroid strain from hops: Evidence for viroid speciation by isolation in different host species. Journal of General Plant Pathology, 70(3), 181–187. https://doi.org/10.1007/s10327-004-0105-z
Sieger, G., Hofmann, H., Förtsch, J., Gross, H. J., Randies, J. W., Sänger, H. L., & Riesner, D. (1984). Conformational transitions in viroids and virusoids: Comparison of results from energy minimization algorithm and from experimental data. Journal of Biomolecular Structure and Dynamics, 2(3), 543–571. https://doi.org/10.1080/07391102.1984.10507591
Štajner, N., Radišek, S., Mishra, A. K., Nath, V. S., Matoušek, J., & Jakše, J. (2019). Evaluation of disease severity and global transcriptome response induced by citrus bark cracking viroid, hop latent viroid, and their co-infection in hop (Humulus lupulus L.). International Journal of Molecular Sciences, 20(13), 3154. https://doi.org/10.3390/ijms20133154
Takahashi, T., & Yaguchi, S. (1985). Strategies for preventing mechanical transmission of hop stunt viroid: Chemical and heat inactivation on contaminated tools. Journal of Plant Diseases and Protection, 92(2), 132–137. https://www.jstor.org/stable/pdf/43383023.pdf. Accessed 29 Apr 2024.
Ziegler, A., Kawka, M., Przybys, M., Doroszewska, T., Skomra, U., Kastirr, U., et al. (2014). Detection and molecular analysis of hop latent virus and hop latent viroid in hop samples from Poland. Journal Für Kulturpflanzen, 66(7), 248–254. https://doi.org/10.5073/JFK.2014.07.04
Acknowledgements
The authors wish to thank Dr. Josef Patzak from the Hop Research Institute Co., LTD in Zatec, Czech Republic, for providing viroid-free plants. We are also grateful to Dr. Christiane Krönauer for her detailed review of the manuscript. Special thanks to Dr. Harald Freiherr von Canstein (EON), Florian Weiß (LfL), Susanne Werner and Torsten Hüller (UHOH), and Sivija Žgajner, Marija Grašinar, and Sabina Gobec (IHPS) for their invaluable technical support. The IHPS part of the research was financially supported by the Slovenian Research Agency, grant no. P4-0077.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
Michael Helmut Hagemann: Conceptualization, PCR planning, data processing, sequence assembly, literature research, and manuscript writing. Kathrin Lutz: Planning, conducting, and analyzing compost experiments. Charlotte Treiber: Conducting degradation experiments and RTqPCR analyses. Ute Born: Sample preparation, extraction, and RTqPCR. Johannes Stampfl: Project design and management of biogas plant operations. Sebastjan Radišek: Conducting inoculation experiments, RT-PCR analysis, risk assessment, and manuscript review & editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no conflict of interest. The IHPS part of the research was financially supported by the Slovenian Research Agency, grant no. P4-0077. The UHOH and LfL parts of the research are self-funded.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hagemann, M.H., Treiber, C., Sprich, E. et al. Composting and fermentation: mitigating hop latent viroid infection risk in hop residues. Eur J Plant Pathol (2024). https://doi.org/10.1007/s10658-024-02869-2
Accepted:
Published:
DOI: https://doi.org/10.1007/s10658-024-02869-2