Abstract
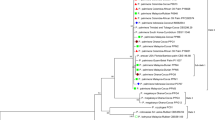
Symptoms of leaf yellowing, reddening, witches’ broom and declining were observed in peach, apricot, and plum orchards from two states and a union territory [Himachal Pradesh (H.P.), Uttarakhand and Jammu & Kashmir (J&K)] of India during 2019–2021. Association of three groups (16SrI, 16SrII,16SrV) of phytoplasma were confirmed in symptomatic peach, plum and apricot samples by amplifying DNA using 16S rRNA and multiple non-ribosomal primers (secA, secY, tuf). Pair wise sequence comparison, phylogenetic analysis and virtual RFLP analysis using multiple non-ribosomal gene sequences confirmed the presence of ‘Candidatus Phytoplasma asteris’ (16SrI-B), ‘Ca. Phytoplasma australasia' (16SrII-D), elm yellows (new subgroup variant of 16SrV) and ‘Ca. Phytoplasma ziziphi' (16SrV-B) related strains in peach, plum and apricot trees. Besides, other suspected symptomatic plant hosts and weeds in and around stone fruit orchards were also identified positive with phytoplasma strains belonging to 16SrI-B and 16Sr II-D subgroups by amplifying 16SrRNA and secA genes and sequence analysis. Association of similar strains of phytoplasma was identified in leafhopper species, Hishimonus phycitis from J&K and Empoasca sp. from Uttarakhand by utilizing the same set of primer pairs and 16S rRNA and secA gene sequence comparison. The presence of ‘Ca. P. asteris’ (16SrI-B) and ‘Ca. P. australasia’ (16SrII-D) related strains detected in peach are new host records from India and the association of elm yellows (a variant of 16SrV subgroup) related strain in plum is the first report in the world.








Similar content being viewed by others
References
Abou-Jawdah, Y., Dakhil, H., Lova, M. M., Sobh, H., Nehme, M., Fakhr-Hammad, E. A., & Bianco, P. A. (2011). Preliminary survey of potential vectors of Candidatus Phytoplasma phoenicium in Lebanon and probability of occurrence of apricot chlorotic leaf roll (ACLR) phytoplasma. Bulletin of Insectology, 64(Supplement), S123–S124.
Abou-Jawdah, Y., Abdel Sater, A., Jawhari, M., Sobh, H., Abdul-Nour, H., Bianco, P. A., & Alma, A. (2014). Asymmetrasca decedens (Cicadellidae, Typhlocybinae), a natural vector of ‘Candidatus Phytoplasma phoenicium. Annals of Applied Biology, 165(3), 395–403.
Ahrens, U., & Seemüller, E. (1992). Detection of DNA of plant pathogenic mycoplasma like organisms by a polymerase chain reaction that amplifies a sequence of the 16S rRNA gene. Phytopathology, 82, 828–832.
Allahverdi, T., Rahimian, H., & Babaeizad, V. (2014). Association of ‘Candidatus Phytoplasma prunorum’ associated with plum yellow leaf stunt in Iran. Iranian Journal Plant Pathology, 50(2), 99.
Balakishiyeva, G., Danet, J. L., Qurbanov, M., Mamedov, A., Kheyr-Pour, A., & Foissac, X. (2010). First report of phytoplasma infections in several temperate fruit trees and vegetable crops in Azerbaijan. Journal of Plant Pathology, 92, S4.115.
Bertaccini, A., & Lee, I.-M. (2018). Phytoplasma: An update. In G. P. Rao, A. Bertaccini, N. Fiore, & L. W. Liefting (Eds.), Phytoplasmas: Plant pathogenic bacteria-I, characterization and epidemiology of phytoplasma-associated diseases (pp. 1–29). Springer.
Bertaccini, A., Duduk, B., Paltrinieri, S., & Contaldo, N. (2014). Phytoplasmas and phytoplasma diseases: A severe threat to agriculture. American Journal of Plant Sciences, 5, 1763–1788.
Blomquist, C. L., & Kirkpatrick, B. C. (2002). Identification of phytoplasma taxa and insect vectors of peach yellow leaf roll disease in California. Plant Disease, 86(7), 759–763.
Cieślińska, M., & Morgaś, H. (2011). Detection and identification of ‘Candidatus Phytoplasma prunorum’, Candidatus Phytoplasma mali’and ‘Candidatus Phytoplasma pyri’ in stone fruit trees in Poland. Journal of Phytopathology, 159(4), 217–222.
Dakhil, H. A., Hammad, E. A. F., El-Mohtar, C., & Abou-Jawdah, Y. (2011). Survey of leafhopper species in almond orchards infected with almond witches’-broom phytoplasma in Lebanon. Journal of Insect Science, 11, 60.
Deng, S., & Hiruki, C. (1991). Amplification of 16S rRNA genes from culturable and nonculturable mollicutes. Journal of Microbiological Methods, 14, 53–61.
FAOSTAT (2019). Agriculture data, agricultural statistics databases. http://faostat.fao.org, Organization of the United Nations, Rome, Italy.
Fiore, N., Bertaccini, A., Bianco, P. A., Cieślińska, M., Ferretti, L., Hoat, T. X., & Quaglino, F. (2018). Fruit crop phytoplasmas. In G. P. Rao, A. Bertaccini, N. Fiore, & L. W. Liefting (Eds.), In Phytoplasmas: Plant pathogenic bacteria-I (pp. 153–190). Springer.
Gundersen, D. E., & Lee, I.-M. (1996). Ultrasensitive detection of phytoplasmas by nested-PCR assays using two universal primer pairs. Phytopathologia Mediterranea, 35, 144–151.
Hemmati, C., Nikooei, M., Al-Subhi, A. M., & Al-Sadi, A. M. (2021). History and current status of phytoplasma diseases in the Middle East. Biology, 10(3), 226.
Hodgetts, J., Boonham, N., Mumford, R., Harrison, N., & Dickinson, M. (2008). Phytoplasma phylogenetics based on analysis of secA and 23S rRNA gene sequences for improved resolution of candidate species of Candidatus Phytoplasma. International Journal of Systematic and Evolutionary Microbiology, 58, 1826–1837.
Khan, J. A., Kumar, J., Thakur, P. D., Handa, A., & Jarial, K. (2013). First report of a 'Candidatus Phytoplasma Ziziphi'-related strain associated with peach decline disease in India. Journal of Plant Pathology, 95, S4–S76.
Kumar, S., Stecher, G., & Tamura, K. (2016). MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Molecular Biology and Evolution, 33(7), 1870–1874.
Lee, I.-M., Zhao, Y., & Bottner, K. D. (2006). SecY gene sequence analysis for finer differentiation of diverse strains in the aster yellows phytoplasma group. Molecular and Cellular Probes, 20(2), 87–91.
Lorenz, K. H., Dosba, F., Poggi-Pollini, C., Llacer, G., & Seemüller, E. (1994). Phytoplasma diseases of Prunus species in Europe are caused by genetically similar organisms. Journal of Plant Diseases and Protection, 101, 567–575.
Marcone, C., Jarausch, B., & Jarausch, W. (2010). ‘Candidatus Phytoplasma prunorum’, the causal agent of European stone fruit yellows: An overview. Journal of Plant Pathology, 92, 19–34.
Marcone, C., Guerra, L. J., & Uyemoto, J. K. (2014). Phytoplasmal diseases of peach and associated phytoplasma taxa. Journal of Plant Pathology, 96(1), 15–28.
Martini, M., Quaglino, F., & Bertaccini, A. (2019). Multilocus genetic characterization of phytoplasma. In A. Bertaccini, K. Oshima, M. Kube, & G. P. Rao (Eds.), Phytoplasmas: Plant pathogenic bacteria-III, genomics, host pathogen interactions and diagnosis (pp. 161–200). Springer.
Marzachi, C., Verati, F., & Bosco, D. (1998). Direct PCR detection of phytoplasmas in experimentally infected insects. Annals of Applied Biology, 153, 45-54.
Mozaffarian, F., & Wilson, M. R. (2016). A checklist of the leafhoppers of Iran (Hemiptera: Auchenorrhyncha: Cicadellidae). Zootaxa, 4062(1), 1–63.
Purcell, A. H. (1982). Insect vector relationships with prokaryotic plant pathogens. Annual Review of Phytopathology, 20, 397–417.
Rao, G. P. (2021). Our understanding about phytoplasma research scenario in India. Indian Phytopathology, 74(5), 371–401.
Rao, G. P., Rao, A., Kumar, M., Ranebennur, H., Mitra, S., & Singh, A. K. (2020). Identification of phytoplasma in six fruit crops in India. European Journal of Plant Pathology, 156(4), 1197–1206.
Salehi, E., & Quaglino, F. (2020). Peach witches’-broom, an emerging disease associated with ‘Candidatus Phytoplasma phoenicium’ and ‘Candidatus Phytoplasma aurantifolia’ in Iran. Crop Protection, 127, 104946.
Schneider, B., Seemüller, E., Smart, C. D., & Kirkpatrick, B. C. (1995). Phylogenetic classification of plant pathogenic mycoplasma-like organisms or phytoplasma. Molecular and Diagnostic Procedures in Mycoplasmology, 1, 369–380.
Schneider, B., Gibb, K. S., & Seemüller, E. (1997). Sequence and RFLP analysis of the elongation factor Tu gene used in differentiation and classification of phytoplasmas. Microbiology, 143(10), 3381–3389.
Singh, J., Rani, A., Kumar, P., Baranwal, V. K., Saroj, P. L., Sirohi, A., Pandey, A. N., & Schenk, P. M. (2014). New host record of a ‘Candidatus Phytoplasma asteris’-related strain infecting peach in India. Australasian Plant Disease Notes, 9(1), 125.
Žežlina, I., Rot, M., Kač, M., & Trdan, S. (2016). Causal agents of stone fruit diseases in Slovenia and the potential for diminishing their economic impact–a review. Plant Protection Science, 52(3), 149–157.
Zhao, Y., Wei, W., Lee, I. M., Shao, J., Suo, X., & Davis, R. E. (2009). Construction of an interactive online phytoplasma classification tool, iPhyClassifier and its application in analysis of the peach X-disease phytoplasma group (16SrIII). International Journal Systematic and Evolutionary Microbiology, 59, 2582–2593.
Zirak, L., Bahar, M., & Ahoonmanesh, A. (2009). Molecular characterization of phytoplasmas related to peanut witches’ broom and “stolbur” groups infecting plum in Iran. Journal of Plant Pathology, 91, 713–716.
Zirak, L., Khakvar, R., Zarrini, G., & Hasanpour, K. (2021). Detection and molecular characterization of phytoplasmas associated with stone fruit trees in northwest of Iran. Crop Protection, 142, 105526.
Zwolińska, A., Borodynko-Filas, N., Nowaczyk, D., & Hasiów-Jaroszewska, B. (2019). First report of Prunus domestica as the host of a phytoplasma belonging to group 16SrI, subgroup B/L. Plant Disease, 103(1), 145–146.
Acknowledgements
The authors are thankful to the Director, ICAR-Indian Agricultural Research Institute, New Delhi, India, for providing financial assistance. Also, wish to express sincere thanks to the Head, Division of Plant Pathology, Indian Agricultural Research Institute, for providing laboratory facilities.
Funding
Consumables funding provided by Indian Agricultural Research Institute, New Delhi, India.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
This paper does not contain studies on human or animal participants.
Informed consent
The paper has not been submitted elsewhere for publication, in whole or in part. Y.S.S. collected the samples and recorded field data and analyses the samples for PCR assays. PVDK analysed the data. AKS, SW and KPS helped in survey of stone fruit crops in different states, GPR drafted the first manuscript and made corrections. All authors contributed to improve the manuscript, reviewed and approved the manuscript.
Conflict of interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Shreenath, Y.S., Singh, A.K., Kumar, P.V.D. et al. Characterization and distribution of phytoplasma strains associated with temperate stone fruits and their possible natural reservoirs in the north-western Himalayan states of India. Eur J Plant Pathol 164, 93–108 (2022). https://doi.org/10.1007/s10658-022-02541-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-022-02541-7