Abstract
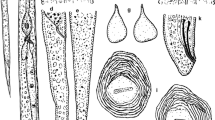
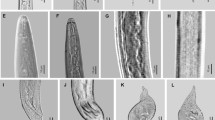
A root-knot nematode (RKN) parasitizing rice (Oryza sativa L.) and causing damage in Santa Catarina (SC), Rio Grande do Sul (RS) and Paraná (PR) states (Brazil) was identified as Meloidogyne ottersoni (Thorne 1969) Franklin 1971. The species is redescribed from the Brazilian population from Meleiro (SC) and compared with the description of M. ottersoni from Wind Lake (Wisconsin, USA) with additional morphological, biochemical and molecular characterization. The female and male bear smaller stylets: 10-12 μm, 14-16 μm, respectively, when compared with M. graminicola: 12-14 μm, 16-18 μm, and M. oryzae: 14-16 μm, 18-20 μm. Meloidogyne ottersoni presents perineal patterns located on the contour of a slight protuberance. Striae are mostly continuous, never raised by transverse irregular striae, as frequently observed in M. graminicola and M. oryzae. Meloidogyne ottersoni belongs to the RKN group 11 described by Jepson (1987); the reproduction is by meiotic parthenogenesis and the somatic chromosome number is 18. The tail of second-stage juveniles is very long and thin, and tapers to a long, narrow, irregular hyaline terminus (M. ottersoni, 20.5 μm vs M. graminicola, 17.9 μm and M. oryzae, 22.0 μm, respectively). The ability of the Brazilian M. ottersoni population to parasitize canary grass, Phalaris arundinacea L. (type host), and barnyard grass, Echinocloa crus-galli, was confirmed. Biochemically, the esterase profile of M. ottersoni lacks any band (Est Ot0, Rm=0), which differentiates it from M. graminicola and M. oryzae (Est VS1, Rm=0.70 and Est O1, Rm=1.02, respectively). In Maximum Likelihood analysis of ITS, D2D3 and COXII-16S rRNA sequences, populations of M. ottersoni from different states of Brazil clustered together and were separated from other Meloidogyne spp., thus confirming that all four populations are very similar and conspecific.









Similar content being viewed by others
References
Adam, M. A. M., Phillips, M. S., & Blok, V. C. (2007). Molecular diagnostic key for identification of single juveniles of seven common and economically important species of root-knot nematode (Meloidogyne spp.). Plant Pathology, 56(1), 190–197.
Bellafiore, S., Jougla, C., Chapuis, É., Besnard, G., Suong, M., Vu, P. N., et al. (2015). Intraspecific variability of the facultative meiotic parthenogenetic root-knot nematode (Meloidogyne graminicola) from rice fields in Vietnam. Comptes Rendus Biologies, 338(7), 471–483.
Bernard, E. C., & Eisenback, J. D. (1997). Meloidogyne trifoliophila n.sp. (Nemata: Meloidogynidae), a parasite of clover from Tennessee. Journal of Nematology, 29(1), 43–54.
Besnard, G., Thi-Phan, N., Ho-Bich, H., Dereeper, A., Trang Nguyen, H., Quénéhervé, P., & Bellafiore, S. (2019). On the close relatedness of two rice-parasitic root-knot nematode species and the recent expansion of Meloidogyne graminicola in Southeast Asia. Genes, 10(2), 175.
Blok, V. C., & Powers, T. O. (2009). Biochemical and molecular identification. In R. N. Perry, M. Moens, & J. L. Starr (Eds.), Root-knot nematodes Wallingford (pp. 98–118). UK: CABI.
Byrd Jr., D. W., Kirkpatrick, T., & Barker, K. R. (1983). An improved technique for clearing and staining plant tissues for detection of nematodes. Journal of Nematology, 15(1), 142.
Carneiro, R. M. D. G., & Almeida, M. R. A. (2001). Técnica de eletroforese usada no estudo de enzimas dos nematoides de galhas para identificação de espécies. Nematologia Brasileira, 25(1), 35–44.
Carneiro, R. M. D. G., Castagnone-Sereno, P., & Dickson, D. W. (1998). Variability among four populations of Meloidogyne javanica from Brazil. Fundamental and Applied Nematology, 4(21), 319–326.
Carneiro, R. M. D. G., Almeida, M. R. A., & Quénéhervé, P. (2000). Enzyme phenotypes of Meloidogyne spp. populations. Nematology, 2(6), 645–654.
Chauhan, B. S., Jabran, K., & Mahajan, G. (2017). Rice Production Worldwide. In R. Prasad, Y. S. Shivay, & D. Kumar (Eds.), Current status, challenges, and opportunities in rice production (pp. 1-15) Springer Nature.
Conab-Companhia Nacional de Abastecimento. Acompanhamento da Safra Brasileira de Grãos (2018), 7(5), 51-60.
De Ley, P., Felix, M. A., Frisse, L. M., Nadler, S. A., Stember, P. W., & Thomas, W. K. (1999). Molecular and morphological characterisation of two reproductive species with mirror image anatomy (Nematoda: Cephalobidae). Nematology, 1(6), 591–612.
De Waele, D., & Elsen, A. (2007). Challenges in tropical plant nematology. Annual Review of Phytopathology, 45(1), 457–485.
Doucet, M. E., & Pinochet, J. (1992). Occurrence of Meloidogyne spp. in Argentina. Supplement to Journal of Nematology, 24(4S), 765–770.
Eisenback, J. D. (1985). Techniques for preparing nematodes for scanning electron microscopy. An advanced treatise on Meloidogyne, 2, 79–105.
Eisenback, J. D., & Hunt, D. J. (2009). General morphology. In R. N. Perry, M. Moens, & J. L. Starr (Eds.), Root-knot nematodes (pp. 18–54). Wallingford, UK: CABI.
Eisenback, J. D., & Triantaphyllou, H. H. (1991). Root-Knot nematode: Meloidogyne spp. and races. In W. R. Nickle (Ed.), Manual of Agricultural Nematology (pp. 191–274). New York: Macel Deccker, Inc..
Esbenshade, P. R., & Triantaphyllou, A. C. (1985). Use of enzyme phenotypes for identification of Meloidogyne species (Nematoda: Tylenchida). Journal of Nematology, 17(1), 6–20.
Franklin, M. T. (1971). Taxonomy of Heteroderidae. In: Zuckerman, B.M., Mai, W.F. and Rohde, R.A. (eds.). Plant Parasitic Nematodes, vol II. Academic Press, New York, pp. 139–162.
Handoo, Z. A., Huettel, R. N., & Golden, A. M. (1993). Description and SEM observations of Meloidogyne sasseri n.sp. (Nematoda: Meloidogynidae), parasitizing beachgrasses. Journal of Nematology, 25(4), 628.
Htay, C., Peng, H., Huang, W., Kong, L., He, W., Holgado, R., & Peng, D. (2016). The development and molecular characterization of a rapid detection method for rice root-knot nematode (Meloidogyne graminicola). European Journal of Plant Pathology, 146(2), 281–291.
Hu, M. & Gasser, R. B. (2006). Mitochondrial genomes of parasitic nematodes – progress and perspectives. Trends in Parasitology, 22(2), 78–84
Hugall, A., Moritz, C., Stanton, J., & Wolstenholme, D. R. (1994). Low, but strongly structured mitochondrial DNA diversity in root knot nematodes (Meloidogyne). Genetics, 136(3), 903–912.
Hugall, A., Stanton, J., & Moritz, C. (1997). Evolution of the AT-rich mitochondrial DNA of the root knot nematode, Meloidogyne hapla. Molecular Biology and Evolution, 14(1), 40–48.
Hunt, D. J., & Handoo, Z. A. (2009). Taxonomy, identification and principal species. In R. N. Perry, M. Moens, & J. L. Starr (Eds.), Root-knot nematodes (pp. 55–97). Wallingford: CABI.
Hussey, R. S., & Barker, K. R. A. (1973). Comparision of methods of collecting inocula of Meloidogyne spp. including a new technique. Plant Disease Reporter, 57(12), 1025–1028.
Jepson, S. B. (1987). Identification of root-knot nematodes (Meloidogyne species). Wallingford: CABI.
Karssen, G., & Moens, M. (2006). Root-knot nematodes Plant Nematology. Wallingford, UK, CABI Publishing.pp. 59-90.
Khan, M. R. (2015). Nematode diseases of crop in India. In L. P. Awasthi (Ed.), Recent advances in the diagnosis and managament of plant diseases (pp. 183–224). New Delhi.pp: Springer.
Mattos, V. S., Soares, M. R. C., Gomes, A. C. M. M., Arieira, C. R. D., Gomes, C. B., & Carneiro, R. M. D. G. (2017). Caracterização de um complexo de espécies do nematoide das galhas parasitando arroz irrigado na região sul do Brasil. In Boletim de Pesquisa e Desenvolvimento 331 (28p). Embrapa Recursos: Genéticos e Biotecnoloiga.
Mattos, V. S., Cares, J. E., Gomes, C. B., Gomes, A. C. M. M., Monteiro, J. D. M. S., Gomez, G. M., Castagnone-Sereno, P., & Carneiro, R. M. D. G. (2018). Integrative Taxonomy of Meloidogyne oryzae (Nematoda: Meloidogyninae) parasitizing rice crops in Southern Brazil. European Jounal of Plant Pathology, 151(3), 649–662.
McClure, M. A., Nischwitz, C., Skantar, A. M., Schmitt, M. E., & Subbotin, S. A. (2012). Root-knot nematodes in golf course greens of the western United States. Plant Disease, 96(5), 635–647.
Moens, M., Perry, R. N., & Starr, J. L. (2009). Meloidogyne species – a diverse group of novel and important plant parasites. In R. N. Perry, M. Moens, & J. L. Starr (Eds.), Root knot nematodes Wallingford (pp. 1–17). UK: CABI.
Negretti, R. R., Gomes, C. B., Mattos, V. S., Somavilla, L., Manica-Berto, R., Agostinetto, D., Castagnone-Sereno, P., & Carneiro, R. M. D. G. (2017). Characterisation of a Meloidogyne species complex parasitising rice in southern Brazil. Nematology, 19(4), 403–412.
Nylander, J. A. A. (2004). MrModelTest, version 2 Evolutionary Biology Centre. Uppsala University.
Padial, J. M., Miralles, A., De La Riva, I., & Vences, M. (2010). The integrative future of taxonomy. Frontiers in Zoology, 7(1), 16.
Pagan, C., Coyne, D., Carneiro, R., Kariuki, G., Luambano, N., Affokpon, A., & Williamson, V. M. (2015). Mitochondrial haplotype-based identification of ethanol-preserved root-knot nematodes from Africa. Phytopathology, 105(3), 350–357.
Pante, E., Abdelkrim, J., Viricel, A., Gey, D., France, S. C., Boisselier, M. C., & Samadi, S. (2015). Use of RAD sequencing for delimiting species. Heredity, 114(5), 450.
Pokharel, R. R., Abawi, G. S., Zhang, N., Duxbury, J. M., & Smart, C. D. (2007). Characterization of isolates of Meloidogyne from rice-wheat production fields in Nepal. Journal of Nematology, 39(3), 221.
Pokharel, R. R., Abawi, G. S., Duxbury, J. M., Smat, C. D., Wang, X., & Brito, J. A. (2010). Variability and the recognition of two races in Meloidogyne graminicola. Australasian Plant Pathology, 39(4), 326–333.
Powers, T. O., & Harris, T. S. (1993). A polymerase chain reaction method for identification of five major Meloidogyne species. Journal of Nematology, 25(1), 1–6.
Rubinoff, D., & Holland, B. S. (2005). Between two extremes: mitochondrial DNA is neither the panacea nor the nemesis of phylogenetic and taxonomic inference. Systematic Biology, 54(6), 952–961.
Salalia, R., Walia, R. N., Somvanshi, V. S., Kumar, P., & Kumar, A. (2017). Morphological , morphometric and molecular characterization of intraspecific variation within Indian populations of Meloidogyne graminicola. Journal of Nematology, 49(3), 254–267.
Schmitz, B., Burgermeister, W., & Braasch, H. (1998). Molecular genetic classification of Central European Meloidogyne chitwoodi and M. fallax populations. Nachrichtenblatt des Deutschen Pflanzenschutzdienstes, 50(12), 310–317.
Subbotin, S., Waeyenberge, A. L., & Moens, M. (2000). Identification of cyst forming nematodes of the genus Heterodera (Nematoda: Heteroderidae) based on the ribosomal DNA RFLPs. Nematology, 2(2), 153–164.
Tamura, K., Peterson, D., Peterson, N., Stecher, G., Nei, M., & Kumar, S. (2011). MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Molecular Biology and Evolution, 28(10), 2731–2739.
Taylor, D. P., & Netscher, C. (1974). An improved technique for preparing perineal patterns of Meloidogyne spp. Nematologica, 20(2), 268–269.
Thorne, G. (1969). Hypsoperine ottersoni sp. n. (Nemata, Heteroderidae) infesting Canary grass, Phalaris arundinacea (L.) reed in Wisconsin. Proceedings of the Helminthological Society of Washington, 36, 98–102.
Triantaphyllou, A. C. (1973). Gametogenesis and reproduction of Meloidogyne graminis and M. ottersoni (Nematoda: Heteroderidae). Journal of Nematology, 5(2), 84.
Triantaphyllou, A. C. (1985). Cytological methods for the study of oogenesis and reproduction of root-knot nematodes. In K. R. Barker, C. C. Carter, & J. N. Sasser (Eds.), An advanced treatise on Meloidogyne: Methodology (Vol. 2, pp. 107–114). Raleigh: North Carolina State University Graphics.
Trifinopoulos, J., Nguyen, L. T., von Haeseler, A., & Minh, B. Q. (2016). W-IQ-TREE: a fast online phylogenetic tool for maximum likelihood analysis. Nucleic Acids Research, 44(W1), W232–W235.
Zijlstra, C., Lever, A. E. M., Uenk, B. J., & van Silfhout, C. H. Y. (1995). Differences between ITS regions of isolates of root-knot nematodes Meloidogyne hapla and M. chitwoodi. Phytopathology, 85(10), 1231–1237.
Acknowledgments
This work was supported by National Council for Scientific an Technological Development (CNPq), Embrapa Genetic Resources and Biotecnology, and Research Foundation of the Federal District (FAPDF). R.R. Leite and R.M.D.G. Carneiro thank CNPq and Coordination of Improvement of Higher Education (CAPES) for their scholarships and the assistant technician Ingrid Gracielle Martins da Silva for the electron microscopy photos and the Microanalysis Laboratory of the Institute of Biological Sciences of the University of Brasilia (LMM-UnB).
Funding
Funding provided by National Council for Scientific and Technological Development (CNPq), Embrapa Genetic Resources and Biotechnology and Research Foundation of the Federal District (FAPDF). Grant numbers:193.001.167/2015
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Human participants and/or animals
Not applicable.
Informed consent
Not applicable.
Rights and permissions
About this article
Cite this article
Leite, R.R., Mattos, V.S., Gomes, A.C.M.M. et al. Integrative taxonomy of Meloidogye ottersoni (Thorne, 1969) Franklin, 1971 (Nematoda: Meloidogynidae) parasitizing flooded rice in Brazil. Eur J Plant Pathol 157, 943–959 (2020). https://doi.org/10.1007/s10658-020-02049-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-020-02049-y