Abstract
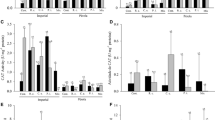
The in vitro ability to produce enzymes and the toxicological influence of culture filtrates from potential fungal plant pathogens on seed germination, sprout growth, stabilization of cell membranes (electrical conductivity of exudates), and growth of seedlings of garden cress (Lepidium sativum L.) were investigated. The fungi used in the study showed significantly different ability to secrete enzymes with different activities. The highest amylolytic activity was shown by Fusarium oxysporum (Fo2), proteolytic activity by F. sulphureum (Fs1564), Gibberella avenacea (Ga2) and G. intricans (Gi2), cellulitic activity by Haematonectria haematococca (Hh776), and pectinolytic activity by strains Fo1 and Fo2 of F. oxysporum. All the fungal filtrates significantly reduced percentages of seed germination, the growth of sprout and seedlings, and destabilization of cell membranes in seedlings. Fusarium poae (Fp2) and G. fujikuroi (Gf1) most strongly reduced seed germination and sprout growth, whereas G. fujikuroi (Gf1) most strongly affected growth and destabilization of cell membranes in seedlings. Fungal enzyme abilities, and in particular pectinase synthesis, do not determine the impact of their culture filtrates on seeds and seedlings. Probably other secondary metabolites synthesized by F. poae (Fp2) and G. fujikuroi (Gf1) have a negative influence on seeds and seedlings of garden cress. The plant used in this study is very sensitive to external factors. Therefore, in the near future, different model plants e.g., cereals, which are less sensitive to external factors, will be used for in-depth characterization of the toxicological influence of culture filtrates from Fusarium spp. and Gibberella spp. on plants.





Similar content being viewed by others
References
Agarwal, N., & Sharma, S. (2013). Garden cress (Lepidium sativum L.) – a non conventional traditional plant item for food product. Indian Journal of Traditional Knowledge, 12, 699–706.
Aoki, T., O’Donnell, K., & Scandiani, M. (2005). Sudden death syndrome of soybean in South America is caused by four species of Fusarium: Fusarium brasiliense sp. nov., F. cuneirostrum sp. nov., F. tucumaniae and F. virguliforme. Mycoscience, 46, 162–183.
Baer, D., & Gudmestad, N. C. (1995). In vitro cellulolytic activity of the plant pathogen Clavibacter michiganensis subsp. sepedonicus. Canadian Journal of Plant Pathology, 41, 877–888.
Bai, G. H., Desjardins, A. E., & Plattner, R. D. (2002). Deoxynivalenol-nonproducing Fusarium graminearum causes initial infection, but does not cause disease spread in wheat spikes. Mycopathologia, 153, 91–98.
Bliss, C. T. (1934). The method of probits. Science, 79, 38–39.
Boot, C. (1971). The genus Fusarium. Kew: Commonwealth Mycological Institute.
Chang, D. C., Grant, G. B., O’Donnell, K., Wannemuehler, K. A., Noble-Wang, J., Rao, C. Y., Jacobson, L. M., Crowell, C. S., Sneed, R. S., Lewis, F. M. T., Schaffzin, J. K., Kainer, M. A., Genese, C. A., Alfonso, E. C., Jones, D. B., Srinivasan, A., Fridkin, S. K., & Park, B. J. (2006). A multistate outbreak of Fusarium keratitis associated with use of a contact lens solution. Journal of the American Medical Association, 296, 953–963.
Coleman, J. J., Rounsley, S. D., Rodriguez-Carres, M., Kuo, A., Wasmann, C. C., Grimwood, J., Schmutz, J., Taga, M., White, G. J., Zhou, S., Schwartz, D. C., Freitag, M., Ma, L.-J., Danchin, E. G. J., Henrissat, B., Coutinho, P. M., Nelson, D. R., Straney, D., Napoli, C. A., Barker, B. M., Gribskov, M., Rep, M., Kroken, S., Molnár, I., Rensing, C., Kennell, J. C., Zamora, J., Farman, M. L., Selker, E. U., Salamov, A., Shapiro, H., Pangilinan, J., Lindquist, E., Lamers, C., Grigoriev, I. V., Geiser, D. M., Covert, S. F., Temporini, E., & VanEtten, H. D. (2009). The genome of Nectria haematococca: contribution of supernumerary chromosomes to gene expansion. PLoS Genetics, 5(8), e1000618. doi:10.1371/journal.pgen.1000618.
Csonka, L. N. (1989). Physiological and genetic responses of bacteria to osmotic stress. Microbiology Reviews, 53, 121–147.
Datta, P. K., Diwakar, B. T., Viswanatha, S., Murthy, K. N., & Naidu, K. A. (2011). Safety evaluation studies on garden cress (Lepidium sativum L.) seeds in Wistar rats. International Journal of Applied Research in Natural Products, 4, 37–42.
Daud, M. E. (1986). Tissue culture and the selection of resistance to pathogens. Annual Review of Phytopathology, 24, 159–186.
Desjardins, A. E., Proctor, R. H., Bai, G. H., McCormick, S. P., Shaner, G., Buchley, G., & Hohn, T. M. (1996). Reduced virulence of trichothecene-nonproducing mutants of Gibberella zeae in wheat field tests. Molecular Plant-Microbe Interactions, 9, 775–781.
Desjardins, A. E., Munkvold, G. P., Plattner, R. D., & Proctor, R. H. (2002). FUM1 – a gene required for fumonisin biosynthesis but not for maize ear rot and ear infection by Gibberella moniliformis in field tests. Molecular Plant-Microbe Interactions, 15, 1157–1164.
Desmond, O. J., Manners, J. M., Stephens, A. E., Maclean, D. J., Schenk, P. M., Gardiner, D. M., Munn, A. L., & Kazan, K. (2008). The Fusarium mycotoxin deoxynivalenol elicits hydrogen peroxide production, programmed cell death and defence responses in wheat. Molecular Plant-Microbe Interactions, 9, 435–445.
Diwakar, B. T., Dutta, P. K., Lokesh, B. R., & Naidu, K. A. (2010). Physicochemical properties of garden cress (Lepidium sativum l.) seed oil. Journal of the American Oil Chemists’ Society, 87, 539–548.
Dobinson, K. F., Lecomte, N., & Lazarovits, G. (1996). Production of an extracelular trypsin-like protease by the fungal plant pathogen Verticillium dahliae. Canadian Journal of Microbiology, 43, 227–233.
Doke, S., & Guha, M. (2014). Garden cress (Lepidium sativum L.) seed - an important medicinal source: a Review. Journal of Natural Product and Plant Resources, 4, 69–80.
Dwivedi, R. (1988). Effect of growth products of staled fungal spp. on germination and field of exotic potato culture. Indian Phytopathology, 41, 500–502.
Eddouks, M., Maghrani, M., Zeggwagh, N. A., & Michel, J. B. (2005). Study of the hypoglycemic activity of Lepidium sativum L. aqueous extract in normal and diabetic rats. Journal of Ethnopharmacology, 97, 391–395.
Fairchild, J. F., Ruessler, D. S., Haverland, P. S., & Carlson, A. R. (1997). Comparative sensitivity of Selenastrum capricornutum and Lemna minor to sixteen herbicides. Archives of Environmental Contamination and Toxicology, 32, 353–357.
Fairchild, J. F., Ruessler, D. S., & Carlson, A. R. (2009). Comparative sensitivity of five species of macrophytes and six species of algae to atrazine, metribuzin, alachlor, and metolachlor. Environmental Toxicology and Chemistry, 17, 1830–1834.
Feng, J., Liu, G., Selvaraj, G., Hughes, G. R., & Wei, Y. (2005). A secreted lipase encoded by LIP1 is necessary for efficient use of saturated triglyceride lipids in Fusarium graminearum. Microbiology, 151, 3911–3921.
Gardiner, D. M., McDonald, M. C., Covarelli, L., Solomon, P. S., Rusu, A. G., Marshall, M., Kazan, K., Chakraborty, S., McDonald, B. A., & Manners, J. M. (2012). Comparative pathogenomics reveals horizontally acquired novel virulence genes in fungi infecting cereal hosts. PLoS Pathogy, 8, e1002952. doi:10.1371/journal.ppat.1002952.
Geiser, D. M., Aoki, T., Bacon, C. W., Baker, S. E., Bhattacharyya, M. K., Brandt, M. E., Brown, D. W., Burgess, L. W., Chulze, S., Coleman, J. J., Correll, J. C., Covert, S. F., Crous, P. W., Cuomo, C. A., De Hoog, G. S., Di Pietro, A., Elmer, W. H., Epstein, L., Frandsen, R. J., Freeman, S., Gagkaeva, T., Glenn, A. E., Gordon, T. R., Gregory, N. F., Hammond-Kosack, K. E., Hanson, L. E., Jímenez-Gasco Mdel, M., Kang, S., Kistler, H. C., Kuldau, G. A., Leslie, J. F., Logrieco, A., Lu, G., Lysøe, E., Ma, L. J., McCormick, S. P., Migheli, Q., Moretti, A., Munaut, F., O’Donnell, K., Pfenning, L., Ploetz, R. C., Proctor, R. H., Rehner, S. A., Robert, V. A., Rooney, A. P., Bin Salleh, B., Scandiani, M. M., Scauflaire, J., Short, D. P., Steenkamp, E., Suga, H., Summerell, B. A., Sutton, D. A., Thrane, U., Trail, F., Van Diepeningen, A., Vanetten, H. D., Viljoen, A., Waalwijk, C., Ward, T. J., Wingfield, M. J., Xu, J. R., Yang, X. B., Yli-Mattila, T., & Zhang, N. (2013). One fungus, one name: defining the genus Fusarium in a scientifically robust way that preserves longstanding use. Phytopathology, 103, 400–408.
Gokavi, S. S., Malleshi, N. G., & Guo, M. (2004). Chemical composition of garden cress (Lepidium sativum) seeds and its fractions and use of bran as a functional ingredient. Plant Foods for Human Nutrition, 59, 105–111.
Haikal, N. Z. (2008). Effect of filtrates of pathogenic fungi of soybean on seed germination and seedling parameters. Journal of Applied Sciences Research, 4, 48–52.
Idris, A. E., Abouzeid, M. A., Boari, A., Vurro, M., & Evidente, A. (2003). Identification of phytotoxic metabolites of a new Fusarium sp. inhibiting germination of Striga hermonthica seeds. Phytopathologia Mediterranea, 42, 65–70.
Ilgen, P., Maier, F., & Schäfer, W. (2008). Trichothecenes and lipases are host-induced and secreted virulence factors of Fusarium graminearum. Cereal Research Communications, 36, 421–428.
Jenczmionka, N. J., & Schäfer, W. (2005). The Gpmk1 MAP kinase of Fusarium graminearum regulates the induction of specific secreted enzymes. Current Genetics, 47, 29–36.
Juma, A. (2007). The effects of Lepidium sativum, a review of contemporary literature and medicimal properties. Oriental Pharmacy and Experimental Medicine, 7, 331–335.
Kang, Z., & Buchenauer, H. (2000a). Ultraestructural and cytochemical studies on cellulose, xylan and pectin degradation in wheat spikes infected by Fusarium culmorum. Journal of Phytopathology, 148, 263–275.
Kang, Z., & Buchenauer, H. (2000b). Cytology and ultrastructure of the infection of wheat spikes by Fusarium culmorum. Mycological Research, 104, 1083–1093.
Khurshid, S., Shoaib, A., & Javaid, A. (2014). In vitro toxicity evaluation of cultire filtrates of Fusarium oxysporum f.sp. lycopersici on growth and physiology of tomato under chromium (VI) stress. The Journal of Animal & Plant Sciences, 24, 1241–1245.
Knogge, W. (1996). Fungal infection of plants. The Plant Cell, 8, 1711–1722.
Ma, L.-J., Geiser, D. M., Proctor, R. H., Rooney, A. P., O’Donnell, K., Trail, F., Gardiner, D. M., Manners, J. M., & Kazan, K. (2013). Fusarium pathogenomics. Annual Review of Microbiology, 67, 399–416.
Madhosing, C. (1995). Relative wilt-inducing capacity of the culture filtrates of isolates of Fusarium oxysporum f.sp. radicis-lycopersici, the tomato crown and root-rot pathogen. Journal of Phytopathology, 4, 193–198.
Maier, U. H., Gundlach, H., & Zenk, M. H. (1998). Seven imidazole alkaloids from Lepidium sativum. Phytochemistry, 49, 1791–1795.
Martínez, M. J., Alconada, T. M., Guillén, F., Vázquez, C., & Reyes, F. (1991). Pectic activities from Fusarium oxysporum f.sp. melonis. Purification and characterization of an exopolygalacturonase. FEMS Microbiology Letters, 81, 145–150.
Marwood, C. A., Solomon, K. R., & Greenberg, B. M. (2001). Chlorophyll fluorescence as a bioindicator of effects on growth in aquatic macrophytes from mixtures of polycyclic aromatic hydrocarbons. Environmental Toxicology and Chemistry, 20, 890–898.
McLean, M. (1996). The phytotoxicity of Fusarium metabolites: an update since 1989. Mycopathologia, 133, 163–179.
Moreira, F. G., dos Reis, S., Costa, M. A. F., de Souza, C. G. M., & Peralta, R. M. (2005). Production of hydrolytic enzymes by the plant pathogenic fungus Myrothecium verrucaria in submerged cultures. Brazilian Journal of Microbiology, 36, 7–11.
Nelson, P. E., Toussoun, T. A., & Marasas, W. F. O. (1983). Fusarium species. An illustrated manual for identification. University Park: The Pennsylvania State University Press.
Nema, A. G. (1992). Studies on pectinolytic and cellulolytic enzymes produced by Fusarium udum causing wilt of Pigeonpea. Indian Journal of Forestry, 15, 353–355.
Nightingale, M. J., Marchylo, B. A., Clear, R. M., Dexter, J. E., & Preston, K. R. (1999). Fusarium head blight: effect of fungal proteases on wheat storage proteins. Cereal Chemistry, 76, 150–158.
Niture, S. K., Kumar, A. R., & Pant, A. (2006). Role of glucose in production and repression of polygalacturonase and pectate lyase from phytopathogenic fungus Fusarium moniliforme NCIM 1276. World Journal of Microbiology and Biotechnology, 22, 893–899.
North, M. J. (1982). Comparative biochemistry of the proteinases of eucaryotic microorganisms. Microbiology Reviews, 46, 308–340.
O’Donnell, K., Kistler, H. C., Cigelnik, E., & Ploetz, R. C. (1998). Multiple evolutionary origins of the fungus causing Panama disease of banana: concordant evidence from nuclear and mitochondrial gene genealogies. Proceedings of the National Academy of Sciences of the United States of America, 95, 2044–2049.
O’Donnell, K., Kistler, H. C., Tacke, B. K., & Casper, H. H. (2000). Gene genealogies reveal global phylogeographic structure and reproductive isolation among lineages of Fusarium graminearum, the fungus causing wheat scab. Proceedings of the National Academy of Sciences of the United States of America, 95, 7905–7910.
O’Donnell, K., Rooney, A. P., Proctor, R. H., Brown, D. W., McCormick, S. P., Ward, T. J., Frandsen, R. J. N., Lysøe, E., Rehner, S. A., Aoki, T., Robert, V. A. R. G., Crous, P. W., Groenewald, J. Z., Kang, S., & Geiser, D. M. (2013). Phylogenetic analyses of RPB1 and RPB2 support a middle Cretaceous origin for a clade comprising all agriculturally and medically important fusaria. Fungal Genetics and Biology, 52, 20–31.
Oeser, B., Heidrich, P. M., Müller, U., Tudzynski, P., & Tenberge, K. B. (2002). Polygalacturonase is a pathogenicity factor in the Claviceps purpurea/rye interaction. Fungal Genetics and Biology, 36, 176–186.
Panda, T., Nair, S. R., & Kumar, P. (2004). Regulation of synthesis of the pectolytic enzymes of Aspergillus niger. Enzyme Microbiology Technics, 34, 466–473.
Pekkarinen, A. I., Mannonen, L., Jones, B. L., & Niku-Paavola, M. L. (2000). Production of proteases by Fusarium species grown on barley grains and in media containing cereal proteins. Journal of Cereal Science, 31, 253–261.
Phalip, V., Delande, F., Carapito, C., Goubet, F., Hatsch, D., Leize-Wagner, E., Dupree, P., Van Dorsselaer, A., & Jetsch, J.-M. (2005). Diversity of the exoproteome of Fusarium graminearum grown on plant cell wall. Current Genetics, 48, 366–379.
Pritsch, C., Muehlbauer, G. J., Bushnell, W. R., Somers, D. A., & Vance, C. P. (2000). Fungal development and induction of defense response genes during early infection of wheat spikes by Fusarium graminearum. Molecular Plant-Microbe Interactions Journal, 13, 159–169.
Raval, N. D., & Pandya, T. N. (2011). Pharmacognostic study of Lepidium sativum Linn (Chandrashura). Ayu, 32, 116–119.
Rehman, N.-U., Khan, A.-U., Alkharfy, K. M., & Gilani, A.-H. (2012). Pharmacological basis for the medicinal use of Lepidium sativum in airways disorders. Evidence-based Complementary and Alternative Medicine, 8, doi: 10.1155/2012/596524.
Roncero, M. I. G., Hera, C., Ruiz-Rubio, M., García-Maceira, F. I., Madrid, M. P., Caracuel, Z., Calero, F., Delgado-Jarana, J., Roldán-Rodriguez, R., & Martínez-Rocha, A. L. (2003). Fusarium as a model for studying virulence in soilborne plant pathogens. Physiological and Molecular Plant Pathology, 62, 87–98.
Sexton, A. C., & Howlett, B. J. (2006). Parallels in fungal pathogenesis on plant and animal hosts. Eukaryotic Cell, 5, 1941–1949.
Sharma, S., Singh, A. K., Singh, R. P., Singh, M. K., Singh, P., & Mohapatra, C. (2015). Effects of plant growth regulator on in vitro callogenesis of Garden cress (Lepidum sativum L.). The Bioscan, 10, 167–171.
Shimokawa, T., Kakegawa, K., & Ishii, T. (2002). Feruloyl esterases from suspension-cultured rice cells. Bulletin FFPRI, 1, 225–230.
Su, L. J., & Arab, L. (2006). Salad and raw vegetable consumption and nutritional status in the adult US population: results from the third national health and nutrition examination survey. Journal of the American Dietetic Association, 106, 1394–1404.
Suthar, R., Bhatt, D. P., & Bhatt, P. N. (2014). Effect of culture filtrate of Fusarium equiseti on seed germination and seedling growth of cumin (Cuminumcyminum). Indian Phytopathology, 67, 193–194.
Sutton, D. A., & Brandt, M. E. (2011). Fusarium and other opportunistic hyaline fungi. In J. Versalovic, K. C. Carroll, G. Funke, J. H. Jorgensen, M. L. Landry, & D. W. Warnock (Eds.), Manual of clinical microbiology (10th ed., pp. 1853–1879). Washington: ASM Press.
Svábová, L., Lebeda, A., Kitner, M., Sedlárová, M., Petrivalsky, M., Dostálová, R., Ondrej, M., Horácek, J., Smykalová, I., & Griga, M. (2011). Comparison of the effects of Fusarium solani in vitro and in vivo on the morphological characteristics and peroxidase activity in pea cultivars with different susceptibility. Journal of Plant Pathology, 93, 19–30.
Tuncay, O., Esiyok, D., Yagmur, B., & Okur, B. (2011). Yield and quality of Garden cress affected by different nitrogen sources and growing period. African Journal of Agriculture Research, 6, 608–617.
Urbanek, H. (1987). The role of the enzyme in the interaction higher plant-pathogen. Wiadomości Botaniczne, 31, 15–28 [in Polish].
Valette-Collet, O., Cimerman, A., Reignault, P., Levis, C., & Boccara, M. (2003). Disruption of Botrytis cinerea pectin methylesterase gene Bcpme1 reduces on several host plans. Molecular Plant-Microbe Interactions Journal, 16, 360–367.
van der Does, C., Duyvesteijn, R. G. E., Goltstein, P. M., van Schie, C. C. N., Manders, E. M. M., Cornelissen, B. J. C., & Repet, M. (2008). Expression of effector gene SIX1 of Fusarium oxysporum requires living plant cells. Fungal Genetic and Biology, 45, 1257–1264.
Wagh, P., Sinha, S., Singh, H. K., & Khare, U. K. (2013). Pathogenic behaviour of Alternaria alternata and phytotoxicity of its culture filtrates on Lepidium sativum: a medicinal herb of immense pharmacological potential. The Bioscan, 8, 643–647.
Walton, J. D. (1994). Deconstructing the cell wall. Plant Physiology, 104, 191–196.
Woloshuk, C. P., & Shim, W.-B. (2013). Aflatoxins, fumonisins, and trichothecenes: a convergence of knowledge. FEMS Microbiology Reviews, 37, 94–109.
Wu, F. (2007). Measuring the economic impacts of Fusarium toxins in animal feeds. Animal Feed Science and Technology, 137, 363–374.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ogórek, R. Enzymatic activity of potential fungal plant pathogens and the effect of their culture filtrates on seed germination and seedling growth of garden cress (Lepidium sativum L.). Eur J Plant Pathol 145, 469–481 (2016). https://doi.org/10.1007/s10658-016-0860-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-016-0860-7