Abstract
As has been observed in several European countries, the frequency of Fusarium head blight (FHB) caused by members of the Fusarium graminearum species complex (FGSC) has increased in Norwegian cereals in recent years, resulting in elevated levels of deoxynivalenol in cereal grains. The objective of this study was to determine if this increase was associated with changes in FGSC composition within Norway. FGSC isolates collected from wheat, oats and barley in Norway during two periods, mainly 1993–1998 and 2004–2007, were characterized to determine species and trichothecene genotype composition and to assess levels of genetic variation and population structure. In vitro growth rates at different temperatures and aggressiveness in spring wheat were further characterized for a sub-selection of isolates. All Norwegian isolates were identified as F. graminearum. The 3-acetyl-deoxynivalenol (3-ADON) trichothecene type was dominant. However, isolates with the 15-ADON chemotype were detected in Norway for the first time and may represent a recent introduction of this trichothecene type. Bayesian-model based clustering and analyses of genetic differentiation indicated the persistence over the last 20 years of two sympatric and partially admixed populations of F. graminearum in Norway. Significant differences in average in vitro growth rates and aggressiveness were observed between these two populations. Our results demonstrate that the recent increase in prevalence of the FGSC in Norwegian cereals do not correspond to any dramatic changes in FGSC species or trichothecene chemotype composition. However, significant changes in population frequencies were observed among Norwegian F. graminearum.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fusarium head blight (FHB) is a disease of small grain cereals, which can cause major losses due to yield reduction and contamination of grain with trichothecenes and other mycotoxins. The global incidence of FHB has increased over the past several decades (Goswami and Kistler 2004) and the International Maize and Wheat Improvement Centre has recognised FHB as a major factor limiting cereal production worldwide (Stack 2000). Because of their ability to inhibit protein synthesis and modify immune function in eukaryotes, trichothecenes pose a significant risk to food and feed safety (Rocha et al. 2005). The global re-emergence of FHB has been linked to the increased adoption of reduced tillage practices and greater precipitation during the growing season, which favour development of FHB pathogens (Bateman et al. 2007; Dill-Macky and Jones 2000; Xu et al. 2005). Similar changes in reduced tillage practices and precipitation during the growing season have been reported from Norway (Tørresen et al. 2012). This may have contributed to the substantial increase in the overall Fusarium spp. infection levels in cereal seeds, as well as the increased levels of trichothecene contamination in cereal grains, observed in Norway in recent years (Bernhoft et al. 2013). During the same period, members of the Fusarium graminearum species complex (FGSC) have become more prevalent in several European countries (Chandelier et al. 2011; Jennings et al. 2004; Stępień et al. 2008; Waalwijk et al. 2003) including the Nordic countries (Bernhoft et al. 2010; Fredlund et al. 2008; Nielsen et al. 2011; Yli-Mattila 2010), partly replacing other Fusarium species.
Members of the FGSC are major agents of FHB worldwide (Goswami and Kistler 2004). Sixteen phylogenetically distinct species have been identified within the FGSC (O’Donnell et al. 2000; O’Donnell et al. 2008; O’Donnell et al. 2004; Starkey et al. 2007; Yli-Mattila et al. 2009; Sarver et al. 2011). F. graminearum has a cosmopolitan distribution (O’Donnell et al. 2000; Backhouse 2014) and is the dominant member of the FGSC in Europe (Laday et al. 2004; O’Donnell et al. 2004; Toth et al. 2005; Yli-Mattila et al. 2009). In fact, F. graminearum has been the only representative of the FGSC reported from Europe with the exception of a small number of F. boothii and F. vorosii isolates from Hungary (Toth et al. 2005; Starkey et al. 2007), a similarly small numbers of F. cortaderiae and F. boothii × F. graminearum hybrids from France (Boutigny et al. 2014), and two F. cortaderiae isolates from Italy (Somma et al. 2014). Species within the FGSC produce type B trichothecenes such as deoxynivalenol (DON) and nivalenol (Aoki et al. 2012). The DON-producing species accumulate primarily 3-acetyldeoxynivalenol (3-ADON) or 15-acetyldeoxynivalenol (15-ADON) in addition to DON (Miller et al. 1991). Variation in trichothecene chemotypes may have important implications for toxicity (Forsell and Pestka 1985; Luongo et al. 2008; Minervini et al. 2004). In addition, chemotype differences have been maintained by selection and may have important consequences for pathogen fitness in different environments (Ward et al. 2002). In Europe, 15-ADON is observed in South and Central European countries (Boutigny et al. 2014; Jennings et al. 2004; Talas et al. 2011; Toth et al. 2005), whereas in north-western parts of Europe, 3-ADON has dominated (Fredlund et al. 2013; Langseth et al. 1999; Nielsen et al. 2012; Yli-Mattila et al. 2009).
Improved understanding of the dramatic species and trichothecene chemotype diversity within the FGSC has led to the recognition of recent regional changes in FGSC composition. In Denmark, Nielsen et al. (2012) suggested that 15-ADON was introduced along with F. graminearum around the year 2000, and was more prevalent than 3-ADON in wheat, whereas the opposite was true in oats and barley. In North America, putatively introduced populations of F. graminearum with the 3-ADON type have been replacing the resident 15-ADON population in some regions (Gale et al. 2007; Ward et al. 2008). In Louisiana (Gale et al. 2011) and Uruguay (Umpiérrez-Failache et al. 2013), where F. graminearum with the 15-ADON chemotype is dominant, significant NIV-producing populations of F. asiaticum were recently detected on wheat in major rice-producing areas. In China, F. asiaticum of the 3-ADON type is replacing NIV producing F. asiaticum in some regions (Zhang et al. 2012). In the Canadian and Chinese studies, phenotypic differences were noted that could explain the spread of a novel population and a rapid shift in population frequencies. In Canada, Ward et al. (2008) observed that the emerging 3-ADON populations had higher fecundity (conidia production), larger conidia spores, higher growth rates, as well as higher production of DON compared to the 15-ADON population. In China, F. asiaticum of the 3-ADON type were rapidly replacing populations characterized by the NIV type and were more aggressive on wheat, more toxigenic, produced more and larger conidia, had higher growth rates, and were resistant to higher concentrations of benzimidazole (Zhang et al. 2010, 2012). These authors speculated that the observed phenotypic differences could provide fitness advantages to a population resulting in a range expansion and significant changes in population frequencies. Collectively, these studies have revealed significant changes in FGSC composition that may have consequences for cereal production and food safety. The major objective of our study was to determine if the recent increase in FGSC prevalence in Norwegian cereals was accompanied by changes in the genetic and phenotypic diversity of these pathogens collected over a time period of 20 years.
Material and methods
Isolate collection
One-hundred and twenty-six single spore isolates morphologically identified as members of the F. graminearum species complex were used in this study (Table 1). One-hundred and five of these isolates were of Norwegian origin. Forty-seven of the Norwegian isolates were obtained from existing culture collections at The Norwegian Veterinary Institute and Bioforsk Plant Health and Plant Protection (Norway) and were collected between 1982 and 1998 (defined as old isolates). In total, 25 of these isolates were from oats, 15 isolates were from barley, and five from wheat. Data on the host plant was not available for two of the isolates. Fifty-eight isolates were obtained from cereal grain samples collected at Bioforsk in the period 2004–2007 (new isolates). In total, 39 of these isolates were from wheat seeds, 17 from oats, and one from barley. Data on the host plant was not available for one of the isolates. For comparison, FGSC isolates from Germany (a total of nine isolates; seven old, two new), Finland (a total of four isolates, all old), and Russia (a total of eight isolates; five old, three new) were included in the study. The isolates of German origin were kindly sent us from Ludwig Niessen, Technical University of Munich, Germany and Paul Nicolson, John Innes Centre, UK. The isolates of Finnish and Russian origin were sent to us from Tapani Yli-Matila, University of Turku, Finland. Details about the Finish, Russian and German isolates were previously published (Carter et al. 2002; Yli-Mattila et al. 2009). More details regarding the isolate collections are presented in Table 1. The original culture names are indicated for those single spore isolates that originated from existing cultures.
Genetic characterization
MLGT
All 126 of the FGSC isolates were analysed using a multilocus genotyping assay (MLGT) for identification of species and trichothecene genotype as described by Ward et al. (2008) with additional species probes described by O’Donnell et al. (2008), Sarver et al. (2011), and Yli-Mattila et al. (2009). DNA extraction was performed as described by Umpiérrez-Failache et al. 2013.
In vitro production of trichothecenes was analysed for three 3-ADON and three 15-ADON genotypes using liquid chromatography coupled to a linear ion trap mass spectrometer. Since the two ADON isomers are practically impossible to separate by chromatographic methods, spectra from fragmentation (MS2/MS3) of the [M+acetate]− ions of a putative acetylated DON in the culture extracts were compared with those of authentic standards. The most obvious difference in the fragmentation spectra between a 3- and 15-acetylated DON was the presence of a prominent −30 Da product ion in the spectra of the former most likely due to cleavage of the CH2OH sidegroup attached to C-6. Such a fragment ion was absent in the spectra of 15-ADON, and thus this feature could be used to distinguish between the two isomers.
VNTR
Analysis of population structure was conducted for all 126 isolates using the variable number of tandem repeat (VNTR) markers described by Suga et al. (2004) at locus HK913, HK1043, HK1073, HK967, HK977, HK1059, HK630, and HK957. Data acquisition was performed as described by Ward et al. (2008). Resulting fragments were scored relative to an internal size standard using an ABI3100 Genetic Analyzer with GENEMAPPER 3.7 software (Applied Biosystems).
Data analysis
Analysis of genetic structure within Norwegian F. graminearum isolates was conducted using STRUCTURE version 2.3 (Pritchard et al. 2000). The analyses were based on VNTR allele size data. We used the admixture model with correlated allele frequencies, values of K from 1 to 10, and 100 000 iterations based on the Markov Chain Monte Carlo (MCMC) method after a burn-in period of 100 000. The appropriate value of K was evaluated according to ΔK (Evanno et al. 2005), calculated from 20 runs of each K. Average gene diversity (H), mean number of pairwise differences (PWD), genetic differentiation at the population level (FST- based genetic distance), and analysis of molecular variance (AMOVA) was estimated within Norwegian F. graminearum using Arlequin version 2.0 (Schneider et al. 2000). Pairwise FST distance estimates were calculated based on the number of different alleles. The statistical significance of FST distances was assessed using permutation tests with 1000 permutations. Differences in H and PWD were tested using a one sided two-sample t-test in MiniTab version 16 (Minitab Inc 2009), with ‘Groups within population 1 have H or PWD equal to the groups within population 2’ as the null hypotheis, and ‘Groups within population 1 have lower H or PWD than the groups within population 2’ as the alternative hypothesis. Calculations of pair-wise genetic distances for haploid data (Huff et al. 1993), followed by a principal coordinate analysis (PCA) of the distance matrix (Orloci 1978), were used to further evaluate the major genetic patterns within Norwegian F. graminearum isolates, as well as for the Norwegian isolates compared to a selection of European F. graminearum isolates. Calculations of pair-wise genetic distances and PCA were performed in GenAlEx version 6.5 (Peakall and Smouse 2006, 2012). The genetic differentiation between our Norwegain F. graminearum populations and the Canadian F. graminearum populations described by Ward et al. (2008) was assesed by pairwise FST as described above.
The difference in the relative proportions of new isolates among the Norwegian F. graminearum populations was tested using Fisher’s exact test (Minitab Inc 2009) with ‘The relative proportions of new isolates are equal in both populations’ as the null hypotheis and ‘The relative proportion of new isolates differs between the two populations’ as the alternative hypothesis. The difference in the relative proportion of population 2 isolates in the new versus the old Norwegian F. graminearum collections was tested accordingly. The associations beween host and populaion, or host and age of collection, were tested using a Chi-Square test (Minitab Inc 2009) with ‘There is no association between host and population (or age of collection)’ as the null hypothesis, and ‘There is an association between host and population (or age of collection)’ as the alternative hypothesis. Where significant associations were observed, the results were further interpreted on the basis of Chi-square contributions.
Phenotypic traits
In vitro fungal growth
In vitro growth of 21 Norwegian F. graminearum isolates was measured on PDA (potato dextrose agar) at four temperatures. The isolates were selected based on MLGT identification of these isolates as F. graminearum and based on a preliminary AFLP analysis to ensure that different genotypes were included in the analysis of phenotypic traits. Nine centimetre petri dishes with PDA were inoculated with agar plugs (5 mm) of actively growing mycelium and were sealed with parafilm. The diameters of the fungal colonies were measured after 1 and 7 days of growth in darkness at 10 and 15 °C, and after 1 and 3 days of growth in darkness at 20 and 25 °C. Within each replicate of the experiment, the growth rate for each fungal isolate was calculated as the average daily radial growth of mycelium within the time period between the two growth measurements in three replicate dishes. The experiment was conducted three times.
Production of perithecia on carrot agar was evaluated at 15, 20 and 25 °C for these 21 isolates as previously described (Gilbert et al. 2008). This experiment was not repeated.
Aggressiveness
Aggressiveness was evaluated for the same group of F. graminearum isolates as used in the growth rate study. Spring wheat cultivar Zebra were sown in 19-cm pots (seven plants per pot) containing a fertilized mixture of sand and peat (P-jord from LOG containing 70 vol% sphagnum peat H2-H4 + 20 vol% sphagnum peat H6-H8 + 10 vol% sand, N = 950 mg l−1, P-cat = 40 mg l−1, K-cat = 180 mg l−1). The plants were grown in a greenhouse for about 2 months at 15/10 °C day/night temperature, 60–80 % relative humidity and 16 h additional light (Osram Powerstar HQ i-BT 400 W/D, at 150–200 μmol PAR m−2 s−1). At heading the day/night temperature was adjusted to 20/15 °C and the period of additional light increased to 18 h. The plants were fertilized weekly with a nutrient solution composed of ‘Superba rød’ (YARA, Mg 4.2, S 5.7, B 0.03, Cu 0.01, Fe 0.18, Mn 0.07, Mo 0.007, Zn 0.037 weight percentage) and Calcium Nitrate (YARA) adjusted to a final concentration of 1.5 to 1.9 mS cm−1.
For each replicate, inoculum consisting of a spore suspension of F. graminearum was prepared and stored at −20 °C a few days prior to use. The inoculum was produced by growing F. graminearum on mung bean agar (MBA) at 20–23 °C and 12 h white light + NUV (50 μmol PAR m−2 s−1) for about 2 weeks. MBA was prepared as described previously (Dill-Macky 2003). Conidia were then washed off the agar surface with distilled water, and one ml of spore suspension (5 × 105 conidia ml−1) was transferred to new MBA plates. After 11 days of growth at the same temperature and light conditions as above, conidia were washed off the agar surface and diluted in distilled water to a final inoculum concentration of 1 × 105 conidia ml−1.
The wheat plants were inoculated at flowering. For each of the 21 selected F. graminearum isolates, spikes within four separate pots were inoculated within each replicate of the experiment. Within each pot, one spikelet on 10 separate wheat heads at the same growth stage were inoculated with a 10 μl droplet of a F. graminearum spore suspension by using a micropipette. Distilled water was used in the non-inoculated control. After inoculation, the wheat heads were covered with moistened plastic bags for 48 h. The plants were further grown in a greenhouse under the same conditions as prior to inoculation. Disease symptoms were scored as the number of bleached spikelets from the inoculation point and downwards in each head at 1.5, 2.5 and 3.5 weeks after inoculation. The four replicate pots for each isolate were placed randomly at separate locations in the greenhouse room both during cultivation and after inoculation. The experiment was repeated tree times.
Data analysis
In order to assess differences in in vitro growth rates between F. graminearum isolates, average growth rates recorded for each isolate within each experiment were subjected to statistical analysis. Within each replicate of the experiment, there were three petri dishes of each isolate within each temperature regime. The experiment was conducted three times. In the third replicate of the experiment, the temperature regime at 25 °C was excluded from the data analysis as the temperature was not stable throughout the experiment. To study differences in aggressiveness between F. graminearum isolates, the average numbers of bleached spikelets per spike registered below the point of inoculation after 3.5 weeks of incubation for each isolate of F. graminearum within experiment were used in the statistical analysis. The experiment was conducted three times and within each replicate of the experiment, 40 spikes were inoculated within each isolate.
Significant differences in growth rates or aggressiveness between individual isolates were separated by applying analysis of variance based on the general linear model (GLM), creating pairwise comparisons and 95 % confidence intervals according to Tukey’s method in MiniTab version 16 (Minitab Inc 2009). Significant differences in growth rates, or aggressiveness, between groups of isolates (population 1 versus population 2, 15-ADON versus 3-ADON genotypes, new versus old isolates) were separated by applying analysis of variance based on GLM and Tukey’s method in MiniTab version 16, including replicate experiment, age, trichothecene genotypes, and population as factors in the model.
Results
Species identification and trichothecene genotypes
Using the MLGT assay, all isolates were identified as F. graminearum. The trichothecene genotypes of the 126 F. graminearum isolates included in this study are presented in Table 1. The vast majority (101) of the 105 Norwegian F. graminearum isolates had 3-ADON genotypes. Only four isolates (ID 200594, 200632, 200650 and 200837) were identified with 15-ADON genotypes, and these were all isolated in 2006 or 2007. Chemotype predictions based on trichothecene genotypes were confirmed by in vitro analyses of trichothecene metabolite profiles for three isolates of each chemotype. Among the F. graminearum isolates of German, Finish or Russian origin, eight had 3-ADON genotypes and 13 were identified with 15-ADON genotypes.
Genetic characterization
Genetic diversity
The eight primer combinations used for VNTR analysis resulted in 60 alleles with sizes in the range of 131–357 base pairs. PCA analysis based on VNTR data from 126 F. graminearum isolates revealed a sub-division of isolates that was connected to chemotype differences (Fig. 1). This analysis grouped 16 of the 17 15-ADON isolates together with three 3-ADON isolates (the latter were one Norwegian and two Russian isolates). The same group also contained 14 of the 17 German and Russian isolates, and all Norwegian 15-ADON isolates. The remaining group consisted of 99 % of the Norwegian 3-ADON isolates together with two German isolates (one 15-ADON and one 3-ADON), one Russian 3-ADON isolate, and all four Finish isolates (all 3-ADON). The first three principal coordinates accounted for 49.4, 17.2, and 13.1 % of the total variation.
Principal coordinate analysis of VNTR data from 126 isolates of F. graminearum from Norway (circles, 105), Finland (diamonds, 4), Germany (triangles, 9) and Russia (squares, 8). Filled symbols indicate 15-ADON genotypes, and open symbols indicate 3-ADON genotypes. The dashed line indicates the main separation between the two groups of trichothecene genotypes in the figure
Genetic structure within Norwegian F. graminearum
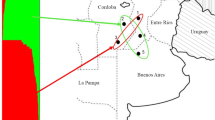
VNTR data were further analyzed with STRUCTURE software to identify possible genetic clusters among the Norwegian F. graminearum isolates. Although K = 5 resulted in the highest log probability of the data (−439) K = 2 resulted in the highest ΔK (>20). ΔK for any of the other tested K values was less than 12. K = 2 had a higher log probability of the data (−542) than K = 1 (−638). Therefore, it was concluded that division of the Norwegian isolates into two genetic clusters captured the major genetic structure in our data set, with higher log probabilities associated with K = 5 possibly reflecting lower-level substructure in the data. Assignment of each Norwegian F. graminearum isolate to one of the two populations derived from Bayesian-model based clustering are presented in Table 1. Population 1 was predominant (59.1 %) among all the isolates tested. Note that all four of the 15-ADON isolates from Norway were assigned to population 2. The two population clusters didn’t correspond to clear differences in demographic data, including host, period or location of geographic origin (results not shown), and the lack of correspondence between genetic structure and demographic data was confirmed by AMOVA and FST analyses (results not shown). Interestingly, the frequencies of these two populations changed significantly between the old (1982–1998) and new (2004–2007) sampling periods (P < 0.005). Population 1 predominated among the older isolate collection (76.6 %), whereas population 2 accounted for a majority of the isolates from the newer isolate collection (55.2 %). There was a significant association between the age of the collections and host, as a higher relative frequency of wheat, and a lower relative frequency of barley was observed in the new compared to the old isolate collection (p < 0.001). However, no association was observed between population and host (p = 0.070). Isolates from all locations were represented in both populations (Fig. 2). These results demonstrated that both populations co-existed at the same locations and on the same hosts during the same time periods.
Geographic distribution of 101 Norwegian F. graminearum isolates. The number of isolates in the New (N) and Old (O) groups within each population is indicated as number of isolates in population 1/number of isolates in population 2. Populations were defined by STRUCTURE software. Four Norwegian F. graminearum isolates were not included in the figure due to unknown origin
The presence of genetic structure was supported by a high fixation index (FST of 0.34, p < 0.001). A high degree of variation among (31.5 %) and within (66 %) the two populations was identified via AMOVA (Table 2). Sub-division of old and new groups of isolates within each population (i.e. Old-1 versus New-1 and Old-2 versus New-2) was almost absent (2.2 % of the total variation). This was further supported by high pairwise FST values between temporal groups from different populations (FST in the range 0.28–0.45, all significant at p < 0.001) (Table 3), whereas FST values between groups within each population were low and not significant (F ST < 0.04, p > 0.08). Correspondingly, the two populations were separated in the PCA analysis, whereas the temporal groups within each population were overlapping (Fig. 3). The two populations were less differentiated within the new isolates (FST of 0.28) than within old isolates (FST of 0.45), consistent with admixture reducing the genetic differentiation between the populations over time.
PWD and H were significantly higher in groups within population 2 (PWD of 2.75–3.45 and H of 0.34–0.43) than for the groups within population one (PWD of 1.39–1.75 and H of 0.17–0.22; p ≤ 0.05), except for average genetic diversity in Old-2 (H of 0.34) and New-1 (H of 0.22) (Table 4). Groups within each population were not statistically different in terms of H and PWD (p > 0.05). The analysis of genetic differentiation between Norwegian and Canadian F. graminearum populations resulted in FST values >0.3 (p < 0.001), indicating no close relationship between these populations.
Phenotypic traits
Eleven of the 62 Norwegian F. graminearum isolates in population 1, and 10 of the 43 Norwegian F. graminearum isolates in population 2, including all four 15-ADON genotypes, were analysed in the phenotypic studies.
In vitro fungal growth
The average radial mycelial growth rate on PDA increased with increasing temperature for all except one (200835) of the 21 F. graminearum isolates (Fig. 4a). Increasing the incubation temperature from 10 to 25 °C had negligible influence on average growth rate for fungal colonies of this isolate, and it was therefore not included in the statistical analyses. The average growth rates (and standard deviations) across the remaining F. graminearum isolates were 3.2 (0.6), 4.6 (0.8), 8.4 (2.7) and 13.7 (3.3) mm per day at 10, 15, 20 and 25 °C respectively (Fig. 4a). Significant positive correlations ranging from 0.82 to 0.92 were observed when comparing growth rates of F. graminearum isolates at different temperatures (results not shown). Growth rates were significantly associated with population affiliation at all temperatures (Table 5), whereas the age of isolates (old versus new) were only significantly associated with growth rate at 25 °C. Lower average growth rates were recorded for isolates in population 2 compared to population 1 at all temperatures (Table 5). Reduced average growth rates were also recorded for isolates in population 2 versus population 1 when only 3-ADON isolates were included (data not shown). The average growth rates were significantly associated with trichothecene genotypes at 20 and 25 °C (Table 5). Three out of the four 15-ADON isolates had a growth rate below the average growth rate calculated across isolates within each growth temperature regime (Fig. 4a), and significantly reduced average growth rates were registered for 15-ADON compared to 3-ADON isolates at 20 and 25 °C (Table 5). All isolates produced perithecia on carrot agar. Additional experiments will be required to determine if there were differences in the amount of perithecia produced.
Growth rate at 10 °C (diamonds), 15 °C (circles), 20 °C (triangles), and 25 °C (squares) (a) and aggressiveness (Aggr.) (b) of Fusarium graminearum isolates. Dashed lines in figure a indicate mean growth rate at 10 °C (lowermost), 15, 20 and 25 °C (uppermost part of figure). Dark indicators or bars in figure b designate isolates in population 2, light indicators or bars designate isolates in population 1. Asterisks designate isolates with15-ADON genotypes
Aggressiveness
Significant differences in aggressiveness between the individual F. graminearum isolates were detected at 3.5 weeks after inoculation. At this time point, average aggressiveness on spring wheat differed from 0.9 to 7 bleached spikelets per spike between the least (200732) and the most (200646) aggressive isolate (Fig. 4b). A similar range of aggressiveness was observed between isolates collected in the period prior to 1999 (old isolates) and isolates collected after year 2003 (new isolates). According to Tukey’s method at a 95.0 % confidence interval, the aggressiveness in old compared to new isolates was not significantly different (results not shown). Isolates 200630, 200650, 200646 and 200736 had a significantly higher aggressiveness than isolates 200732 and 200726, while no significant differences in aggressiveness were registered between the remaining isolates according to Tukey’s method at a 95.0 % confidence interval. No significant difference in average disease development (average number of bleached spikelets per spike of 4.1) was registered between the three replicate experiments at 3.5 weeks after inoculation.
To study the association between genotypic traits and aggressiveness, data on 21 isolates were included in the statistical analysis. Aggressiveness was significantly associated with both trichothecene genotype and population affiliation (p < 0.002), whereas no significant effect was detected for isolates’ age or for replicate of the experiment. Significantly higher average aggressiveness was registered for population 1 compared to population 2 (Table 5). Higher average aggressiveness was also registered for population 1 compared to population 2 when only 3-ADON isolates were included in the analysis (data not shown). The 15-ADON genotypes included in this study had an aggressiveness ≥4.1 (which was the average aggressiveness across all isolates measured after 3.5 weeks of incubation) (Fig. 4b), and significantly higher average aggressiveness was registered for 15-ADON compared to 3-ADON isolates (Table 5).
No significant correlations were registered between the degree of aggressiveness on wheat and mycelial growth rate on PDA at any of the temperatures for the 20 F. graminearum isolates included in both analyses (results not shown).
Discussion
In the present study, we characterized genetic and phenotypic diversity of Norwegian FGSC isolates and evaluated species composition, trichothecene genotype diversity, and population structure over a 20 year period. Our data indicate that F. graminearum is the only significant species within the FGSC in Norway, and point to the persistence of substantial population structure in Norwegian F. graminearum for at least 20 years, although significant differences in population frequencies and evidence of admixture were noted. In addition, these analyses documented the presence of 15-ADON genotypes of F. graminearum for the first time in Norway.
Species and trichothecene diversity
The present work is the first study of FGSC species diversity in Norway. All FGSC isolates collected from Norwegian cereals were identified as F. graminearum, which is consistent with previous evidence that F. graminearum is the dominant species within this species complex in Europe (Laday et al. 2004; O’Donnell et al. 2004; Toth et al. 2005; Yli-Mattila et al. 2009; Talas et al. 2011; Boutigny et al. 2014). F. graminearum is also dominant in the United States and Canada (Starkey et al. 2007; Gale et al. 2007; Ward et al. 2008; Schmale et al. 2011), and is found on all continents with the exception of Antarctica (O’Donnell et al. 2004; Aoki et al. 2012; Backhouse 2014). While the species within the FGSC show a significant biogeographic structure (Aoki et al. 2012), substantial species diversity has been observed in South America (Scoz et al. 2009; Sampietro et al. 2010; Umpiérrez-Failache et al. 2013), Asia (Yli-Mattila et al. 2009; Suga et al. 2008; Zhang et al. 2012), Africa (Boutigny et al. 2011; O’Donnell et al. 2008) and New Zealand (Monds et al. 2005). Long-distance dispersal and anthropogenic activities have the potential to alter regional pathogen composition as suggested by the recent discovery of significant populations of F. asiaticum, an Asian endemic that appears to be adapted to rice agroecosystems (Lee et al. 2009), on wheat in major rice production areas of Louisiana and Uruguay (Gale et al. 2011). However, climatic conditions in Norway and other parts of Northern Europe may create a barrier to the establishment of some FGSC species. A recent analysis of FGSC pathogen distributions on wheat in relation to climate found significant differences between F. graminearum, F. boothii, and F. asiaticum in the ranges of most climate parameters (Backhouse 2014). This analysis indicated that the distributions of F. asiaticum and F. boothii may be restricted by climatic conditions to a much greater extent than is the case for F. graminearum. However, similar analyses for the other members of the FGSC are lacking due to insufficient distribution information, and changes in climate or cropping practices could increase the potential for other FGSC species to become established in Norway.
The overwhelming majority of F. graminearum on cereals in Norway have 3-ADON genotypes. Correspondingly, 3-ADON, but not 15-ADON was detected in wheat, oats, and barley collected at Norwegian grain elevators in 2011 (Uhlig et al. 2013). The 3-ADON chemotype also predominated among a small set of FGSC isolates from an earlier collection of Norwegian cereals (Langseth et al. 1999, 2001), and was found in oat and wheat samples collected between 2009 and 2011 in Sweden, whereas the 15-ADON chemotype was not observed (Fredlund et al. 2013; Lindblad et al. 2013). Previous multilocus genotype analysis indicated that F. graminearum from Finland and Northwestern Russia were exclusively 3-ADON (Yli-Mattila et al. 2009), and PCR assays indicated that 3-ADON genotypes were dominant in barley, triticale, rye, and oat in Denmark, although 15-ADON genotypes were dominant in wheat collected between 2003 and 2007 in Denmark (Nielsen et al. 2012). Our data add to a growing set of studies indicating a geographical partitioning of trichothecene types (chemotypes or genotypes) throughout Europe, with 3-ADON dominating in Northern Europe, whereas 15-ADON appears predominant in the Western and Southern Europe including the UK (Jennings et al. 2004), Belgium (Audenaert et al. 2009), Italy (Prodi et al. 2009; Somma et al. 2014), Luxembourg (Pasquali et al. 2010), Poland (Stępień et al. 2008), Hungary (Toth et al. 2005), Germany (Talas et al. 2011), and France (Boutigny et al. 2014). In addition, isolates with NIV genotypes were absent from our sample, but account for at least 10 % of the FGSC isolates in some other parts of Europe (Jennings et al. 2004; Waalwijk et al. 2003; Boutigny et al. 2014; Stępień et al. 2008; Somma et al. 2014).
The distribution of trichothecene types is likely influenced directly or indirectly by a variety of factors including climate, regional host composition, agricultural practices, and the recent and historical movement of pathogen populations into new areas. A significant longitudinal cline in trichothecene chemotype frequencies in Canada was attributed to the recent introduction and spread of a novel, and potentially more vigorous, F. graminearum population with a 3-ADON chemotype (Ward et al. 2008). A very similar situation was described for F. asiaticum populations differing in trichothecene type in China (Zhang et al. 2010, 2012). Recent introductions may also account for regional differences in trichothecene chemotype composition reported in Louisiana and Uruguay, although regional differences in host composition were suggested as a major factor (Gale et al. 2011; Umpiérrez-Failache et al. 2013). The association between trichothecene chemotype differences and host composition was also suggested by a study in Canada reporting the prevalence of 15-ADON genotypes of F. graminearum from wheat and maize, whereas only 3-ADON genotypes were isolated from potatoes (Burlakoti et al. 2012). Host-specific differences in trichothecene genotype frequencies were also noted in Denmark (Nielsen et al. 2012). In contrast, regional differences in the prevalence of NIV genotypes in France were not correlated with differences in host species, suggesting that trichothecene chemotype differences did not play a major role in shaping host associations in that study.
While 3-ADON genotypes were dominant in our study, we detected four isolates with 15-ADON genotypes. The 15-ADON isolates were obtained from the two most recent years that we examined (2006 and 2007), and no 15ADON isolates were observed in the older collection of isolates dating back to 1982. It is possible that F. graminearum with the 15-ADON trichothecene type have been present at low frequency in Norway for a long period of time and their absence from our older isolate collection is due to sampling artifacts. If 15-ADON types are preferentially associated with wheat as indicated by a recent study in Denmark (Nielsen et al. 2012) the relatively lower proportion of isolates from wheat in our older collection also may have contributed to a failure to sample 15-ADON types among older isolates. However, isolates producing 15-ADON were also absent in previous surveys of Norwegian isolates (Langseth et al. 1999, 2001), whereas in vitro production of 15-ADON was confirmed for three of the 15-ADON genotypes collected in our survey. In adition, our PCA analyses (Fig. 1) suggested a close connection between Norwegian isolates with the 15-ADON type and those from other parts of Europe. We interpret these results as indicating that strains with the 15-ADON type were recently introduced into Norway, and their association with population 2 in the Bayesian clustering analysis is likely an artefact owing to the very low frequency of these novel isolates within our Norwegian isolate collection. Nielsen et al. (2012) observed a dominance of 3-ADON in Danish small grain cereals and did not detect 15-ADON isolates until 1997, even though samples collected from as far back as 1957 were included in that analysis. The authors speculated that 15-ADON genotypes were introduced into Denmark along with F. graminearum around the year 2000 and are now the dominant type observed on wheat in that country. Infected seeds represent a risk for introducing pathogens into new areas, and imported seed lots may have introduced 15-ADON into Norway. In addition, long distance dispersal of viable F. graminearum spores is possible (Maldonado-Ramirez et al. 2005; Schmale et al. 2012). We speculate whether the increased prevalence of FGSC observed in several European countries (Chandelier et al. 2011; Jennings et al. 2004; Stępień et al. 2008; Waalwijk et al. 2003) including the Nordic countries (Bernhoft et al. 2010; Fredlund et al. 2008; Nielsen et al. 2011; Yli-Mattila 2010) may have facilitated the introduction of the 15-DON genotypes into new areas like Norway and Denmark. As such, the prevalence of 15-ADON genotypes and associated mycotoxin levels in Norwegian cereals should be monitored.
In our analyses, isolates with 15-ADON genotypes exhibited higher levels of aggressiveness than were observed among 3-ADON isolates. However, larger numbers of strains will need to be analyzed before conclusions can be drawn about the potential significance of trichothecene chemotype variation in Norway. From a food safety perspective, the acetylated derivatives of DON are rapidly deacetylated in vivo (Eriksen and Pettersson 2004), such that DON will be the major toxin found in grain infected with either 3-ADON or 15-ADON producers. However, potential strain or population-specific differences in the total amount of trichothecene accumulated in grain is also of concern. In analyses of Canadian F. graminearum, 3-ADON strains accumulated significantly more DON in vivo than 15-ADON strains (von der Ohe et al. 2010; Ward et al. 2008). The prevalence of 3-ADON among Norwegian F. graminearum could therefore indicate a relatively larger threat to food safety in comparison to regions in which 15-ADON isolates predominate. However, trichothecene accumulation potential may be a population-specific feature, not tied directly to trichothecene type, and we demonstrated that the Norwegian 3-ADON producers are not closely related to the North American 3-ADON populations. Additional research is required to understand the toxin accumulation potential of Norwegian F. graminearum in relation to F. graminearum populations outside of Norway.
Population structure and phenotypic diversity
Two populations of F. graminearum were identified within Norway and were shown to co-exist over a time period of at least 20 years. The results provided no evidence of geographic or host partitioning of these two populations, indicating that both populations are widespread in Norway and can be found on the same hosts. Although these two populations remain distinct, substantial reductions in Fst over time indicate that admixture is breaking down the observed population structure. The origin of this population structure is unclear, but may have resulted from the introduction and spread of a novel population prior to our sampling. Similar conclusions were drawn from the observation of widespread co-occurrence of divergent F. graminearum populations in North America (Gale et al. 2007; Ward et al. 2008) and from the observed population structure within F. asiaticum in China (Zhang et al. 2010, 2012).
In addition to observing the widespread co-occurrence of two F. graminearum populations in Norway, we detected a significant shift in population frequencies, with population 2 increasing from 23 % in the older sampling period to more than 50 % in the more recent sampling period (even when 15-ADON isolates were excluded from the analysis). Such a shift could indicate that population 2 has a general adaptive advantage over population 1 or that recent conditions in Norway favour population 2. However, in contrast to the examples from North America and China, the observed population shift in Norway was not readily explainable in terms of observed phenotypic differences between populations. In fact, population 2 displayed lower average radial growth rates and lower average aggressiveness on spring wheat than was observed for population 1. A variety of features are likely involved in determining relative fitness, and it is possible that additional research will reveal phenotypic differences that might explain the observed shift in population frequencies. Alternatively, population 2 may represent an introduced population with its increased frequency owing to increased inoculum pressure from outside of Norway. In this context, the decreased Fst values between the two Norwegian populations may reflect the genetic swamping of population 1 by an introduced population. However, such a scenario would likely produce some geographic structure, which we did not observe. It is also possible that the population frequency shift, while statistically significant, is the product of random fluctuations in frequencies. Our current sampling represents only two points in time and additional monitoring will be required to determine if the observed shift is reflective of a sustained trend.
In Norway, the increased prevalence of the FGSC has coincided with increased adoption of reduced tillage practices and slight changes in weather conditions (Tørresen et al. 2012). The average precipitation during the flowering period of spring cereals (i.e. July) in the main cereal production districts (Østlandet) has gradually increased over the last 20–30 years, and has been accompanied by a slight temperature increase of 1–2 °C above normal (10.8 °C in July) during the last 10 years (Norwegian Meteorological Institute 2013). Incidence of FHB is related to rainfall and temperature during the period close to anthesis (Madgwick et al. 2011). Temperatures above 12 °C facilitates the formation of perithecia by members of the FGSC (Dufault et al. 2006). Thus, in addition to being more favorable for FHB development, the present weather conditions during cereal flowering in Norway appear more conducive for perithecia and ascospore formation by members of the FGSC. This is indeed supported by our recent studies, which demonstrated production of perithecia and ascospores of F. graminearum on inoculated maize residues under field conditions in Norway (unpublished). Although we did not observe any differences in perithecia production between the two F. graminearum populations, a more thorough study is needed to identify fitness advantages associated with the population frequency shift identified in our study.
Conclusion
In Norway, the increased prevalence of the FGSC has coincided with increased adoption of reduced tillage practices and more frequent occurrence of weather conditions favorable for FHB development. Our analyses indicate that these changes have not been associated with significant changes in FGSC species or trichothecene genotype composition within Norway. However, isolates with the 15-DON chemotype were detected for the first time in Norway, and our analyses suggest these isolates were recently introduced from other parts of Europe. In addition, we detected substantial population structure within Norway, which was associated with significant differences in aggressiveness and in vitro growth rate. Additional monitoring will be required to determine if the shift in population frequencies that we observed is indicative of a sustained trend. Understanding the combination of factors responsible for the observed shift in population frequencies and regional differences in trichothecene chemotype composition may enable a prediction of future population distribution. An implementation of relevant measures to reduce introduction and spread of novel pathogens will reduce costs for agriculture and enhance the food safety.
References
Aoki, T., Ward, T., Kistler, H., & O’Donnell, K. (2012). Systematics, phylogeny and trichothecene mycotoxin potential of fusarium head blight cereal pathogens. Mycotoxins, 62(2), 91–102.
Audenaert, K., Van Broeck, R., Bekaert, B., De Witte, F., Heremans, B., Messens, K., et al. (2009). Fusarium head blight (FHB) in Flanders: population diversity, inter-species associations and DON contamination in commercial winter wheat varieties. European Journal of Plant Pathology, 125(3), 445–458.
Backhouse, D. (2014). Global distribution of Fusarium graminearum, F. asiaticum and F. boothii from wheat in relation to climate. European Journal of Plant Pathology, 139, 161–173.
Bateman, G. L., Gutteridge, R. J., Gherbawy, Y., Thomsett, M. A., & Nicholson, P. (2007). Infection of stem bases and grains of winter wheat by Fusarium culmorum and F. graminearum and effects of tillage method and maize-stalk residues. Plant Pathology, 56(4), 604–615.
Bernhoft, A., Clasen, P. E., Kristoffersen, A. B., & Torp, M. (2010). Less Fusarium infestation and mycotoxin contamination in organic than in conventional cereals. Food Additives and Contaminants: Part A, Chemistry, Analysis, Control, Exposure and Risk Assessment, 27(6), 842–852.
Bernhoft, A., Eriksen, G.S., Sundheim, L., Berntssen, M., Brantsæter, A., Brodal, G., et al. (2013). Risk assessment of mycotoxins in cereal grain in Norway. Opinion of the Scientific Steering Committee of the Norwegian Scientific Committee for Food Safety (VKM). http://www.vkm.no/dav/eee04d10c4.pdf.
Boutigny, A.-L., Ward, T. J., Van Coller, G. J., Flett, B., Lamprecht, S. C., O’Donnell, K., et al. (2011). Analysis of the Fusarium graminearum species complex from wheat, barley and maize in South Africa provides evidence of species-specific differences in host preference. Fungal Genetics and Biology, 48(9), 914–920.
Boutigny, A.-L., Ward, T. J., Ballois, N., Iancu, G., & Ioos, R. (2014). Diversity of the Fusarium graminearum species complex on French cereals. European Journal of Plant Pathology, 138(1), 133–148.
Burlakoti, R. R., Tamburic-Ilincic, L., Limay-Rios, V., Peters, R. D., & Burlakoti, P. (2012). Profiling trichothecene genotypes of Fusarium graminearum isolated from corn, wheat, potatoes, in eastern Canada. In US National Fusarium Headblight Forum, Orlando, USA, December 2012.
Carter, J. P., Rezanoor, H. N., Holden, D., Desjardins, A. E., Plattner, R. D., & Nicholson, P. (2002). Variation in pathogenicity associated with the genetic diversity of Fusarium graminearum. European Journal of Plant Pathology, 108(6), 573–583.
Chandelier, A., Nimal, C., André, F., Planchon, V., & Oger, R. (2011). Fusarium species and DON contamination associated with head blight in winter wheat over a 7-year period (2003–2009) in Belgium. European Journal of Plant Pathology, 130, 1–12.
Dill-Macky, R. (2003). Inoculation methods and evaluation of Fusarium head blight resistance in wheat. In K. J. Leonard & W. R. Bushnell (Eds.), Fusarium head blight of wheat and barley (pp. 184–210). St. Paul: The American Phytopathological Society.
Dill-Macky, R., & Jones, R. (2000). The effect of previous crop residues and tillage on Fusarium head blight of wheat. Plant Disease, 84(1), 71–76.
Dufault, N., De Wolf, E., Lipps, P., & Madden, L. (2006). Role of temperature and moisture in the production and maturation of Gibberella zeae perithecia. Plant Disease, 90(5), 637–644.
Eriksen, G. S., & Pettersson, H. (2004). Toxicological evaluation of trichothecenes in animal feed. Animal Feed Science and Technology, 114(1–4), 205–239.
Evanno, G., Regnaut, S., & Goudet, J. (2005). Detecting the number of clusters of individuals using the software STRUCTURE: a simulation study. Molecular Ecology, 14(8), 2611–2620.
Forsell, J. H., & Pestka, J. J. (1985). Relation of 8-ketotrichothecene and zearalenone analog structure to inhibition of mitogen-induced human lymphocyte blastogenesis. Applied and Environmental Microbiology, 50(5), 1304–1307.
Fredlund, E., Gidlund, A., Olsen, M., Borjesson, T., Sphid, N. H. H., & Simonsson, M. (2008). Method evaluation of Fusarium DNA extraction from mycelia and wheat for down-stream real-time PCR quantification and correlation to mycotoxin levels. Journal of Microbiological Methods, 73(1), 33–40.
Fredlund, E., Gidlund, A., Sulyok, M., Börjesson, T., Krska, R., Olsen, M., et al. (2013). Deoxynivalenol and other selected Fusarium toxins in Swedish oats–occurrence and correlation to specific Fusarium species. International Journal of Food Microbiology, 167(2), 276–283.
Gale, L. R., Ward, T. J., Balmas, V., & Kistler, H. C. (2007). Population subdivision of Fusarium graminearum sensu stricto in the upper Midwestern United States. Phytopathology, 97(11), 1434–1439.
Gale, L. R., Harrison, S. A., Ward, T. J., O’Donnell, K., Milus, E. A., Gale, S. W., et al. (2011). Nivalenol-type populations of Fusarium graminearum and F. asiaticum are prevalent on wheat in southern Louisiana. Phytopathology, 101(1), 124–134.
Gilbert, J., Woods, S. M., & Kromer, U. (2008). Germination of ascospores of Gibberella zeae after exposure to various levels of relative humidity and temperature. Phytopathology, 98(5), 504–508.
Goswami, R. S., & Kistler, H. C. (2004). Heading for disaster: Fusarium graminearum on cereal crops. Molecular Plant Pathology, 5(6), 515–525.
Huff, D. R., Peakall, R., & Smouse, P. E. (1993). RAPD variation within and among natural populations of outcrossing buffalograss Buchloe dactyloides (Nutt.) Engelm. Theoretical and Applied Genetics, 86(8), 927–934.
Jennings, P., Coates, M. E., Walsh, K., Turner, J. A., & Nicholson, P. (2004). Determination of deoxynivalenol- and nivalenol-producing chemotypes of Fusarium graminearum isolated from wheat crops in England and Wales. Plant Pathology, 53(5), 643–652.
Laday, M., Juhasz, A., Mule, G., Moretti, A., Szecsi, A., & Logrieco, A. (2004). Mitochondrial DNA diversity and lineage determination of European isolates of Fusarium graminearum (Gibberella zeae). European Journal of Plant Pathology, 110(5–6), 545–550.
Langseth, W., Bernhoft, A., Rundberget, T., Kosiak, B., & Gareis, M. (1999). Mycotoxin production and cytotoxicity of Fusarium strains isolated from Norwegian cereals. Mycopathologia, 144(2), 103–113.
Langseth, W., Ghebremeskel, M., Kosiak, B., Kolsaker, P., & Miller, D. (2001). Production of culmorin compounds and other secondary metabolites by Fusarium culmorum and F. graminearum strains isolated from Norwegian cereals. Mycopathologia, 152(1), 23–34.
Lee, J., Chang, I.-Y., Kim, H., Yun, S.-H., Leslie, J. F., & Lee, Y.-W. (2009). Genetic diversity and fitness of Fusarium graminearum populations from rice in Korea. Applied and Environmental Microbiology, 75(10), 3289–3295.
Lindblad, M., Gidlund, A., Sulyok, M., Börjesson, T., Krska, R., Olsen, M., et al. (2013). Deoxynivalenol and other selected Fusarium toxins in Swedish wheat—Occurrence and correlation to specific Fusarium species. International Journal of Food Microbiology, 167(2), 284–291.
Luongo, D., De Luna, R., Russo, R., & Severino, L. (2008). Effects of four Fusarium toxins (fumonisin B1, α-zearalenol, nivalenol and deoxynivalenol) on porcine whole-blood cellular proliferation. Toxicon, 52(1), 156–162.
Madgwick, J. W., West, J. S., White, R. P., Semenov, M. A., Townsend, J. A., Turner, J. A., et al. (2011). Impacts of climate change on wheat anthesis and fusarium ear blight in the UK. European Journal of Plant Pathology, 130(1), 117–131.
Maldonado-Ramirez, S. L., Schmale, D. G., Shields, E. J., & Bergstrom, G. C. (2005). The relative abundance of viable spores of Gibberella zeae in the planetary boundary layer suggests the role of long-distance transport in regional epidemics of Fusarium head blight. Agricultural and Forest Meteorology, 132(1–2), 20–27.
Miller, J. D., Greenhalgh, R., Wang, Y. Z., & Lu, M. (1991). Trichothecene Chemotypes of 3 Fusarium Species. Mycologia, 83(2), 121–130.
Minervini, F., Fornelli, F., & Flynn, K. (2004). Toxicity and apoptosis induced by the mycotoxins nivalenol, deoxynivalenol and fumonisin B1 in a human erythroleukemia cell line. Toxicology in Vitro, 18(1), 21–28.
Minitab Inc. (2009). Minitab statistical software, release 16 for Windows, State College, Pennsylvania.
Monds, R. D., Cromey, M. G., Lauren, D. R., Di Menna, M., & Marshall, J. (2005). Fusarium graminearum, F. cortaderiae and F. pseudograminearum in New Zealand: molecular phylogenetic analysis, mycotoxin chemotypes and co-existence of species. Mycological Research, 109(4), 410–420.
Nei, M. (1987). Molecular evolutionary genetics. New York: Columbia University Press.
Nielsen, L. K., Jensen, J. D., Nielsen, G. C., Jensen, J. E., Spliid, N. H., Thomsen, I. K., et al. (2011). Fusarium head blight of cereals in Denmark: species complex and related mycotoxins. Phytopathology, 101(8), 960–969.
Nielsen, L. K., Jensen, J. D., Rodríguez, A., Jørgensen, L. N., & Justesen, A. F. (2012). TRI12 based quantitative real-time PCR assays reveal the distribution of trichothecene genotypes of F. graminearum and F. culmorum isolates in Danish small grain cereals. International Journal of Food Microbiology, 157(3), 384–392.
Norwegian Meteorological Institute. (2013). Østlandet siden 1900. http://www.met.no/Klima/Klimautvikling/Klima_siste_150_ar/Regioner/Ostlandet/. Accessed 10 January 2013.
O’Donnell, K., Kistler, H. C., Tacke, B. K., & Casper, H. H. (2000). Gene genealogies reveal global phylogeographic structure and reproductive isolation among lineages of Fusarium graminearum, the fungus causing wheat scab. Proceedings of the National Academy of Sciences of the United States of America, 97(14), 7905–7910.
O’Donnell, K., Ward, T. J., Geiser, D. M., Kistler, H. C., & Aoki, T. (2004). Genealogical concordance between the mating type locus and seven other nuclear genes supports formal recognition of nine phylogenetically distinct species within the Fusarium graminearum clade. Fungal Genetics and Biology, 41(6), 600–623.
O’Donnell, K., Ward, T. J., Aberra, D., Kistler, H. C., Aoki, T., Orwig, N., et al. (2008). Multilocus genotyping and molecular phylogenetics resolve a novel head blight pathogen within the Fusarium graminearum species complex from Ethiopia. Fungal Genetics and Biology, 45(11), 1514–1522.
Orloci, L. (1978). Multivariate analysis in vegetation research. The Hague: Dr W. Junk B. V.
Pasquali, M., Giraud, F., Brochot, C., Cocco, E., Hoffmann, L., & Bohn, T. (2010). Genetic Fusarium chemotyping as a useful tool for predicting nivalenol contamination in winter wheat. International Journal of Food Microbiology, 137(2), 246–253.
Peakall, R., & Smouse, P. E. (2006). GENALEX 6: genetic analysis in Excel. Population genetic software for teaching and research. Molecular Ecology Notes, 6(1), 288–295.
Peakall, R., & Smouse, P. E. (2012). GenAlEx 6.5: genetic analysis in Excel. Population genetic software for teaching and research-an update. Bioinformatics, 28, 2537–2539.
Pritchard, J. K., Stephens, M., & Donnelly, P. (2000). Inference of population structure using multilocus genotype data. Genetics, 155(2), 945–959.
Prodi, A., Tonti, S., Nipoti, P., Pancaldi, D., & Pisi, A. (2009). Identification of deoxynivalenol and nivalenol producing chemotypes of Fusarium graminearum isolates from durum wheat in a restricted area of northern Italy. Journal of Plant Pathology, 91(3), 727–731.
Rocha, O., Ansari, K., & Doohan, F. M. (2005). Effects of trichothecene mycotoxins on eukaryotic cells: a review. Food Additives and Contaminants, 22(4), 369–378.
Sampietro, D. A., Marín, P., Iglesias, J., Presello, D. A., Vattuone, M. A., Catalan, C. A. N., et al. (2010). A molecular based strategy for rapid diagnosis of toxigenic Fusarium species associated to cereal grains from Argentina. Fungal Biology, 114(1), 74–81.
Sarver, B. A., Ward, T. J., Gale, L. R., Broz, K., Corby Kistler, H., Aoki, T., et al. (2011). Novel Fusarium head blight pathogens from Nepal and Louisiana revealed by multilocus genealogical concordance. Fungal Genetics and Biology, 48(12), 1096–1107.
Schmale, D. G., Wood-Jones, A. K., Cowger, C., Bergstrom, G. C., & Arellano, C. (2011). Trichothecene genotypes of Gibberella zeae from winter wheat fields in the eastern USA. Plant Pathology, 60(5), 909–917.
Schmale, D. G., Ross, S. D., Fetters, T. L., Tallapragada, P., Wood-Jones, A. K., & Dingus, B. (2012). Isolates of Fusarium graminearum collected 40–320 meters above ground level cause Fusarium head blight in wheat and produce trichothecene mycotoxins. Aerobiologia, 28(1), 1–11.
Schneider, S., Roessli, D., & Excoffier, L. (2000). Arlequin: a software for population genetics data analysis, version 2.000. Switzerland: Genetics and Biometry Laboratory, Department of Anthropology, University of Geneva.
Scoz, L., Astolfi, P., Reartes, D., Schmale, D., III, Moraes, M., & Del Ponte, E. (2009). Trichothecene mycotoxin genotypes of Fusarium graminearum sensu stricto and Fusarium meridionale in wheat from southern Brazil. Plant Pathology, 58(2), 344–351.
Somma, S., Petruzzella, A., Logrieco, A., Meca, G., Cacciola, O., & Moretti, A. (2014). Phylogenetic analyses of Fusarium graminearum strains from cereals in Italy, and characterisation of their molecular and chemical chemotypes. Crop and Pasture Science, 65(1), 52–60.
Stack, R. W. (2000). Return of an old problem: Fusarium head blight of small grains. Plant Health Progress. https://www.apsnet.org/publications/apsnetfeatures/Pages/headblight.aspx.
Starkey, D. E., Ward, T. J., Aoki, T., Gale, L. R., Kistler, H. C., Geiser, D. M., et al. (2007). Global molecular surveillance reveals novel Fusarium head blight species and trichothecene toxin diversity. Fungal Genetics and Biology, 44(11), 1191–1204.
Stępień, Ł., Popiel, D., Koczyk, G., & Chełkowski, J. (2008). Wheat-infectingFusarium species in Poland—their chemotypes and frequencies revealed by PCR assay. Journal of Applied Genetics, 49(4), 433–441.
Suga, H., Gale, L. R., & Kistler, H. C. (2004). Development of VNTR markers for two Fusarium graminearum clade species. Molecular Ecology Notes, 4(3), 468–470.
Suga, H., Karugia, G. W., Ward, T., Gale, L. R., Tomimura, K., Nakajima, T., et al. (2008). Molecular characterization of the Fusarium graminearum species complex in Japan. Phytopathology, 98(2), 159–166.
Tajima, F. (1983). Evolutionary relationship of DNA sequences in finite populations. Genetics, 105(2), 437–460.
Tajima, F. (1993). Measurement of DNA polymorphism. In N. Takahata & A. G. Clark (Eds.), Mechanisms of molecular evolution (pp. 37–59). Tokyo: Japan Scientific Societies Press.
Talas, F., Parzies, H. K., & Miedaner, T. (2011). Diversity in genetic structure and chemotype composition of Fusarium graminearum sensu stricto populations causing wheat head blight in individual fields in Germany. European Journal of Plant Pathology, 131(1), 39–48.
Tørresen, K., Hofgaard, I. S., Eklo, O. M., Netland, J., Brandsæter, L. O., Brodal, G., et al. (2012). Redusert jordarbeiding og konsekvenser for plantevern. Bioforsk RAPPORT, 7(58), 1–67.
Toth, B., Mesterhazy, A., Horvath, Z., Bartok, T., Varga, M., & Varga, J. (2005). Genetic variability of central European isolates of the Fusarium graminearum species complex. European Journal of Plant Pathology, 113(1), 35–45.
Uhlig, S., Eriksen, G. S., Hofgaard, I. S., Krska, R., Beltrán, E., & Sulyok, M. (2013). Faces of a changing climate: semi-quantitative multi-mycotoxin analysis of grain grown in exceptional climatic conditions in Norway. Toxins, 5(10), 1682–1697.
Umpiérrez-Failache, M., Garmendia, G., Pereyra, S., Rodríguez-Haralambides, A., Ward, T., & Vero, S. (2013). Regional differences in species composition and toxigenic potential among Fusarium head blight isolates from Uruguay indicate a risk of nivalenol contamination in new wheat production areas. International Journal of Food Microbiology, 166(1), 135–140.
von der Ohe, C., Gauthier, V., Tamburic-Ilincic, L., Brule-Babel, A., Fernando, W., Clear, R., et al. (2010). A comparison of aggressiveness and deoxynivalenol production between Canadian Fusarium graminearum isolates with 3-acetyl and 15-acetyldeoxynivalenol chemotypes in field-grown spring wheat. European Journal of Plant Pathology, 407–417.
Waalwijk, C., Kastelein, P., de Vries, I., Kerenyi, Z., van der Lee, T., Hesselink, T., et al. (2003). Major changes in Fusarium spp. in wheat in the Netherlands. European Journal of Plant Pathology, 109(7), 743–754.
Ward, T. J., Bielawski, J. P., Kistler, H. C., Sullivan, E., & O’Donnell, K. (2002). Ancestral polymorphism and adaptive evolution in the trichothecene mycotoxin gene cluster of phytopathogenic Fusarium. Proceedings of the National Academy of Sciences of the United States of America, 99(14), 9278–9283.
Ward, T. J., Clear, R. M., Rooney, A. P., O’Donnell, K., Gaba, D., Patrick, S., et al. (2008). An adaptive evolutionary shift in Fusarium head blight pathogen populations is driving the rapid spread of more toxigenic Fusarium graminearum in North America. Fungal Genetics and Biology, 45(4), 473–484.
Xu, X. M., Parry, D. W., Nicholson, P., Thomsett, M. A., Simpson, D., Edwards, S. G., et al. (2005). Predominance and association of pathogenic fungi causing Fusarium ear blight in wheat in four European countries. European Journal of Plant Pathology, 112(2), 143–154.
Yli-Mattila, T. (2010). Ecology and evolution of toxigenic Fusarium species in Northern Europe and Asia. Journal of Plant Pathology, 92(1), 7–18.
Yli-Mattila, T., Gagkaeva, T., Ward, T. J., Aoki, T., Kistler, H. C., & O’Donnell, K. (2009). A novel Asian clade within the Fusarium graminearum species complex includes a newly discovered cereal head blight pathogen from the Russian Far East. Mycologia, 101(6), 841–852.
Zhang, H., Zhang, Z., van der Lee, T., Chen, W. Q., Xu, J., Xu, J. S., et al. (2010). Population genetic analyses of Fusarium asiaticum populations from barley suggest a recent shift favoring 3ADON producers in Southern China. Phytopathology, 100(4), 328–336.
Zhang, H., Van der Lee, T., Waalwijk, C., Chen, W., Xu, J., Xu, J., et al. (2012). Population analysis of the Fusarium graminearum species complex from wheat in China show a shift to more aggressive isolates. PloS One, 7(2), e31722.
Acknowledgments
This work was funded by the following partners: the Foundation for Research Levy on Agricultural Products/Agricultural Agreement Research Fund/the Research Council of Norway (research grant 173277/I10 and 199412/I99), the Norwegian cereal industry and Bioforsk. Isolates of F. graminearum were kindly provided by Ludwig Niessen, Technical University of Munich Germany; Paul Nicolson, John Innes Centre, Norwich, UK, and Tapani Yli-Mattila, University of Turku, Finland. We are grateful to Tom Usgaard and Nathane Orwig for excellent technical assistance. The mention of firm names or trade products does not imply that they are endorsed or recommended by the US Department of Agriculture over other firms or similar products not mentioned. The USDA is an equal opportunity provider and employer.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Aamot, H.U., Ward, T.J., Brodal, G. et al. Genetic and phenotypic diversity within the Fusarium graminearum species complex in Norway. Eur J Plant Pathol 142, 501–519 (2015). https://doi.org/10.1007/s10658-015-0629-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-015-0629-4