Abstract
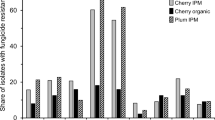
Benzimidazoles, dicarboximides and demethylation inhibitors are the main group of fungicides used to control brown rot in Spain. The causal agents of brown rot in peach (Prunus persica (L.) Batsch) orchards in Spain are Monilinia laxa M. fructigena, and M. fructicola. The dynamics of fungicide sensitivity and fitness of M. fructicola population, the most recent species causing brown rot in the Ebro Valley (Lleida, Spain), were characterized by determining their resistance, fitness, and in vitro competitiveness to thiophanate-methyl (TM), iprodione (I), and cyproconazole (CPZ) in field isolates recovered over the 2006–2010 period. We found that: (a) more than 95 % of the M. fructicola isolates are high TM-resistant, (b) more than 50 % of the M. fructicola isolates are I-resistant and these frequencies of occurrence did not change during our 5-year survey, and (c) a few CPZ-resistant isolates have been also detected in population since 2008. We identified five different fungicide-resistant (R) and/or fungicide-sensitive (S) phenotypes and found that our study population contains multiple fungicide-resistant isolates. Moreover, these fungicide-resistant isolates display high parasitic fitness on fruit and flowers and high competitiveness. These findings suggest that the TM, I, and CPZ resistance of M. fructicola isolates could be contributing as another factor on changing the frequency of occurrence of the three Monilinia species in the Ebro Valley.




Similar content being viewed by others
References
Brent, K. J., & Hollomon, D. W. (2007) Fungicide Resistance: the Assessment of Risk. FRAC Monograph No 2. Global Crop Protection Federation (pp. 23–28). Brussels.
Byrde, R. J., & Willetts, H. J. (1977). The brown rot fungi of fruit. Their biology and control. Oxford: Pergamon Press.
Chen, F., Liu, X., & Schnabel, G. (2013). Field strains of Monilinia fructicola resistant to both MBC and DMI fungicides isolated from stone fruit orchards in the eastern United States. Plant Disease, 97, 1063–1068.
Cox, K. D., Bryson, P. K., & Schnabel, G. (2007). Instability of propiconazole resistance and fitness in Monilinia fructicola. Phytopathology, 97, 448–453.
De Cal, A., & Melgarejo, P. (1999). Effects of long-wave UV light on growth and identification of species. Plant Disease, 83, 62–65.
De Cal, A., Gell, I., Usall, J., Viñas, I., & Melgarejo, P. (2009). First report of brown rot caused by Monilinia fructicola in peach orchards in Ebro Valley, Spain. Plant Disease, 93, 763.
De Cal, A., Egüen, B., & Melgarejo, P. (2014). Vegetative compatibility groups and sexual reproduction among Spanish Monilinia fructicola isolates obtained from peach and nectarine orchards, but not Monilinia laxa. Fungal Biology, 118, 484–494.
EFSA Panel on Plant Health. (2011). Pest risk assessment of Monilinia fructicola for the EU territory and identification and evaluation of risk management options. EFSA Journal, 9(4), 2119.
Elmer, P. A. G., & Gaunt, R. E. (1994). The biological characteristics of dicarboximide resistant isolates of Monilinia fructicola from New Zealand stone fruit orchards. Plant Pathology, 43, 130–137.
Fishel, F. M., & Dewdney, M. M. (2006). Fungicide resistance action committee’s (FRAC): Classification scheme of fungicides according to mode of action. Fla. Coop. Extn. Serv. PI-94. Gainesville: IFAS. Univ. Fla.
Free, S. J., Holtz, B. A., & Michailides, T. J. (1996). Mating behavior in field populations of Monilinia fructicola in California. Mycologia, 88, 208–211.
Gell, I., Cubero, J., & Melgarejo, P. (2007). Two different PCR approaches for universal diagnosis of brown rot and identification of Monilinia spp. in stone fruit trees. Journal of Applied Microbiology, 103, 2629–2637.
Gell, I., De Cal, A., Torres, R., Usall, J., & Melgarejo, P. (2008). Relationship between the incidence of latent infections caused by Monilinia spp. and the incidence of brown rot of peach fruit: factors affecting latent infection. European Journal of Plant Pathology, 121, 487–498.
Grabke, A., Fernández-Ortuño, D., Amiri, A., Li, X., Peres, N. A., Smith, P., & Schnabel, G. (2014). Characterization of iprodione resistance in Botrytis cinerea from strawberry and blackberry. Phytopathology, 104, 396–402.
Holb, I. J., & Schnabel, G. (2007). Differential effect of triazoles on mycelial growth and disease measurements of Monilinia fructicola isolates with reduced sensitivity to DMI fungicides. Crop Protection, 26, 753–759.
Holtz, B. A., Michailides, T. J., & Hong, C. X. (1998). Development of apothecia from stone fruit infected and stromatized by Monilinia fructicola in California. Plant Disease, 82, 1375–1380.
Katan, T., & Shabi, E. (1981). Resistance to dicarboximide fungicides in laboratory isolates of Monilinia fructicola and M. laxa. Netherlands Journal of Plant Pathology, 87, 242.
Larena, I., & Melgarejo, P. (1996). Biological control of Monilinia laxa and Fusarium oxysporum f sp lycopersici by a lytic enzyme-producing Penicillium purpurogenum. Biological Control, 6, 361–367.
Lichtemberg, P. S. F., Zeviani, W. M., Morales, R. G. F., Michailides, T. J., & May-de-Mio, L. L. (2014). Shift in Monilinia fructicola sensitivity to tebuconazole fungicide, and resistance survey in Southern Brazil. In H. W. Dehne, H. B. Deising, B. Fraaije, U. Gisi, D. Hermann, A. Mehl, E. C. Oerke, P. E. Russell, G. Stammler, K. H. Kuck, & H. Lyr (Eds.), Modern Fungicides and Antifungal Compounds VII (pp. 173–178). Braunschweig: Deutsche Phytomedizinische Gesellschaft.
Luo, C. X., & Schnabel, G. (2008). The cytochrome P450 lanosterol 14 alpha-demethylase gene is a demethylation inhibitor fungicide resistance determinant in Monilinia fructicola field isolates from Georgia. Applied and Environmental Microbiology, 74, 359–366.
Ma, Z. H., Yoshimura, M. A., & Michailides, T. J. (2003). Identification and characterization of benzimidazole resistance in Monilinia fructicola from stone fruit orchards in California. Applied and Environmental Microbiology, 69, 7145–7152.
Ma, Z. H., Luo, Y., & Michailides, T. (2006). Molecular characterization of the two-component histidine kinase gene from Monilinia fructicola. Pest Management Science, 62, 991–998.
MAGRAMA 2013. Ministerio de Agricultura Pesca Alimentación y Medio Ambiente. Gobierno de España. “Registro de Productos Fitosanitarios.” http://www.magrama.gob.es.
Malandrakis, A., Markoglou, A. N., & Ziogas, B. N. (2012). PCR-RFLP detection of the E198A mutation conferring resistance to benzimidazoles in field isolates of Monilinia laxa from Greece. Crop Protection, 3, 11–17.
Malandrakis, A., Koukiasas, N., Veloukas, T., Karaoglanidis, G., & Markoglou, A. (2013). Baseline sensitivity of Monilinia laxa from Greece to fenhexamid and analysis of fenhexamid-resistant mutants. Crop Protection, 46, 13–17.
May-de Mio, L. L., Luo, Y., & Michailides, T. J. (2011). Sensitivity of Monilinia fructicola from Brazil to tebuconazole, azoxystrobin, and thiophanate-methyl and implications for disease management. Plant Disease, 95, 821–827.
Michailides, T. J., Ogawa, J. M., & Opgenorth, D. C. (1987). Shift of Monilinia spp and distribution of isolates sensitive and resistant to benomyl in California prune and apricot orchards. Plant Disease, 71, 893–896.
Peever, T. L., & Milgroom, M. G. (1995). Fungicide resistance—lessons for herbicide resistance management? Weed Technology, 9, 840–849.
Penrose, L. J. (1990). Prolonged field persistence of resistance to benomyl in Monilinia fructicola. Crop Protection, 9(3), 190–192.
Pringle, A., & Taylor, J. W. (2002). The fitness of filamentous fungi. Trends in Microbiology, 10, 474–481.
Raposo, R., Colgan, R., Delcan, J., & Melgarejo, P. (1995). Application of an automated quantitative method to determine fungicide resistance in Botrytis cinerea. Plant Disease, 79, 294–296.
Sanoamuang, N., & Gaunt, R. E. (1995). Persistence and fitness of carbendazim-resistant and dicarboximide-resistant isolates of Monilinia fructicola (Wint) Honey in flowers, shoots and fruit of stone fruit. Plant Pathology, 44, 448–457.
Sauer, D. B., & Burroughs, R. (1986). Disinfection of seed surfaces with sodium hypochlorite. Phytopathology, 76, 745–749.
Schnabel, G., Bryson, P. K., Bridges, W. C., & Brannen, P. M. (2004). Reduced sensitivity in Monilinia fructicola to propiconazole in Georgia and implications for disease management. Plant Disease, 88, 1000–1004.
Schoustra, S. E., Slakhorst, M., Debets, A. J. M., & Hoekstra, R. F. (2005). Comparing artificial and natural selection in rate of adaptation to genetic stress in Aspergillus nidulans. Journal of Evolutionary Biology, 18, 771–778.
Thomidis, T., Michailides, T., & Exadaktylou, E. (2009). Contribution of pathogens to peach fruit Rot in northern Greece and their sensitivity to iprodione, carbendazim, thiophanate‐methyl and tebuconazole fungicides. Journal of Phytopathology, 157, 194–200.
Usall, J., De Cal, A., Casals, C., Peris, M., Segarra, J., & Viñas, I. (2010). Control de la podredumbre parda en melocotones y nectarinas del Valle del Ebro. Vida Rural, 302.
Villani, S. M., & Cox, K. D. (2011). Characterizing fenbuconazole and propiconazole sensitivity and prevalence of ‘mona’ in isolates of Monilinia fructicola from New York. Plant Disease, 95, 828–834.
Villarino, M., Larena, I., Martinez, F., Melgarejo, P., & De Cal, A. (2012). Analysis of genetic diversity in Monilinia fructicola from the Ebro Valley in Spain using ISSR and RAPD markers. European Journal of Plant Pathology, 132, 511–524.
Villarino, M., Egüen, B., Lamarca, N., Segarra, J., Usall, J., Melgarejo, P., & De Cal, A. (2013). Occurrence of Monilinia laxa and M. fructigena after introduction of M. fructicola in peach orchards in Spain. European Journal of Plant Pathology, 137, 835–845.
Weger, J., Schanze, M., Hilber-Bodmer, M., Smits, T. H. M., & Patocchi, A. (2011). First report of the beta-tubulin E198A mutation conferring resistance to methyl benzimidazole carbamates in european isolates of Monilinia fructicola. Plant Disease, 95, 497.
Wherrett, A. D., Sivasithamparam, K., & Kumar, S. (2001). Detection of possible systemic fungicide resistance in Western Australian Monilinia populations. Phytopathology, 91, S95.
Wilcox, W. F., & Burr, J. A. (1994). Base-line sensitivity of Monilinia fructicola to six DMI fungicides (Abstr.). Phytopathology, 84, 1078.
Yoshimura, M. A., Luo, Y., Ma, Z. H., & Michailides, T. J. (2004). Sensitivity of Monilinia fructicola from stone fruit to thiophanate-methyl, iprodione, and tebuconazole. Plant Disease, 88, 373–378.
Zhu, F. X., Bryson, P. K., Amiri, A., & Schnabel, G. (2010). First Report of the beta-tubulin E198A allele for fungicide resistance in Monilinia fructicola from South Carolina. Plant Disease, 94, 1511.
Acknowledgments
This study was supported by grants RTA2005-00077-CO2, AGL2008-4396-CO2, and AGL2011-30472-CO2 from the Ministry of Economy and Competitiveness (Spain). B. Egüen received a scholarship from the Spanish Ministry of Economy and Competiveness. We thank R. Castillo and M.T. Morales-Clemente for their technical support, and the growers for their support and collaboration. The authors would like to acknowledge Dr. Arieh Bomzon, ConsulWrite (www.consulwrite.com) for his editorial assistance in preparing this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Egüen, B., Melgarejo, P. & De Cal, A. Sensitivity of Monilinia fructicola from Spanish peach orchards to thiophanate-methyl, iprodione, and cyproconazole: fitness analysis and competitiveness. Eur J Plant Pathol 141, 789–801 (2015). https://doi.org/10.1007/s10658-014-0579-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-014-0579-2