Abstract
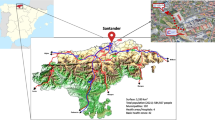
The Rotterdam Study is a population-based cohort study, started in 1990 in the district of Ommoord in the city of Rotterdam, the Netherlands, with the aim to describe the prevalence and incidence, unravel the etiology, and identify targets for prediction, prevention or intervention of multifactorial diseases in mid-life and elderly. The study currently includes 17,931 participants (overall response rate 65%), aged 40 years and over, who are examined in-person every 3 to 5 years in a dedicated research facility, and who are followed-up continuously through automated linkage with health care providers, both regionally and nationally. Research within the Rotterdam Study is carried out along two axes. First, research lines are oriented around diseases and clinical conditions, which are reflective of medical specializations. Second, cross-cutting research lines transverse these clinical demarcations allowing for inter- and multidisciplinary research. These research lines generally reflect subdomains within epidemiology. This paper describes recent methodological updates and main findings from each of these research lines. Also, future perspective for coming years highlighted.

Similar content being viewed by others
References
Hofman A, Grobbee DE, de Jong PT, van den Ouweland FA. Determinants of disease and disability in the elderly: the Rotterdam Elderly Study. Eur J Epidemiol. 1991;7(4):403-22.
Aribas E, Roeters van Lennep JE, De Rijke YB, et al. Sex steroids and sex steroid-binding globulin levels amongst middle-aged and elderly men and women from general population. Eur J Clin Invest. 2022;52(12):e13866.
Aribas E, Kavousi M, Laven JSE, Ikram MA, Roeters van Lennep JE. Aging, Cardiovascular Risk, and SHBG Levels in Men and Women From the General Population. J Clin Endocrinol Metab. 2021;106(10):2890-900.
Aribas E, Ahmadizar F, Mutlu U, et al. Sex steroids and markers of micro- and macrovascular damage among women and men from the general population. Eur J Prev Cardiol. 2022;29(9):1322-30.
Meun C, Gunning MN, Louwers YV, et al. The cardiovascular risk profile of middle-aged women with polycystic ovary syndrome. Clin Endocrinol (Oxf). 2020;92(2):150-8.
Gunning MN, Meun C, van Rijn BB, et al. The cardiovascular risk profile of middle age women previously diagnosed with premature ovarian insufficiency: A case-control study. PLoS One. 2020;15(3):e0229576.
Tilly MJ, Lu Z, Geurts S, et al. Atrial fibrillation patterns and their cardiovascular risk profiles in the general population: the Rotterdam study. Clin Res Cardiol. 2023;112(6):736-46.
Lu Z, Tilly MJ, Geurts S, et al. Sex-specific anthropometric and blood pressure trajectories and risk of incident atrial fibrillation: the Rotterdam Study. Eur J Prev Cardiol. 2022;29(13):1744-55.
Lu Z, Geurts S, Arshi B, et al. Longitudinal Anthropometric Measures and Risk of New-Onset Atrial Fibrillation Among Community-Dwelling Men and Women. Mayo Clin Proc. 2022;97(8):1501-11. doi:S0025-6196(22)00058-1 [pii] https://doi.org/10.1016/j.mayocp.2021.12.018
Lu Z, Tilly MJ, Aribas E, et al. Imaging-based body fat depots and new-onset atrial fibrillation in general population: a prospective cohort study. BMC Med. 2022;20(1):317. doi:10.1186/s12916-022-02505-y [pii] 2505 [pii] https://doi.org/10.1186/s12916-022-02505-y
Geurts S, Brunborg C, Papageorgiou G, Ikram MA, Kavousi M. Subclinical Measures of Peripheral Atherosclerosis and the Risk of New-Onset Atrial Fibrillation in the General Population: the Rotterdam Study. J Am Heart Assoc. 2022;11(1):e023967.
van Kleef LA, Lu Z, Ikram MA, de Groot NMS, Kavousi M, de Knegt RJ. Liver stiffness not fatty liver disease is associated with atrial fibrillation: The Rotterdam study. J Hepatol. 2022;77(4):931-8.
Tilly MJ, Geurts S, Donkel SJ, et al. Immunothrombosis and new-onset atrial fibrillation in the general population: the Rotterdam Study. Clin Res Cardiol. 2022;111(1):96–104.
Roselli C, Chaffin MD, Weng LC, et al. Multi-ethnic genome-wide association study for atrial fibrillation. Nat Genet. 2018;50(9):1225-33.
Siland JE, Geelhoed B, Roselli C, et al. Resting heart rate and incident atrial fibrillation: A stratified Mendelian randomization in the AFGen consortium. PLoS One. 2022;17(5):e0268768.
Geurts S, van der Burgh AC, Bos MM, et al. Disentangling the association between kidney function and atrial fibrillation: a bidirectional Mendelian randomization study. Int J Cardiol. 2022;355:15–22.
van der Burgh AC, Geurts S, Ikram MA, Hoorn EJ, Kavousi M, Chaker L. Bidirectional Association Between Kidney Function and Atrial Fibrillation: A Population-Based Cohort Study. J Am Heart Assoc. 2022;11(10):e025303.
Geurts S, Tilly MJ, Arshi B, et al. Heart rate variability and atrial fibrillation in the general population: a longitudinal and Mendelian randomization study. Clin Res Cardiol. 2023;112(6):747-58.
Arshi B, van den Berge JC, van Dijk B, Deckers JW, Ikram MA, Kavousi M. Implications of the ACC/AHA risk score for prediction of heart failure: the Rotterdam Study. BMC Med. 2021;19(1):43.
Limpens MAM, Asllanaj E, Dommershuijsen LJ, et al. Healthy lifestyle in older adults and life expectancy with and without heart failure. Eur J Epidemiol. 2022;37(2):205-14.
Zhu F, Arshi B, Leening MJG, et al. Sex-specific added value of cardiac biomarkers for 10-year cardiovascular risk prediction. Eur J Prev Cardiol. 2022;29(11):1559-67.
Wolters FJ, Hilal S, Leening MJG, et al. Plasma amyloid-β40 in relation to subclinical atherosclerosis and cardiovascular disease: A population-based study. Atherosclerosis. 2022;348:44–50.
Zhu F, Wolters FJ, Yaqub A, et al. Plasma Amyloid-β in Relation to Cardiac Function and Risk of Heart Failure in General Population. JACC Heart Fail. 2023;11(1):93–102.
Arshi B, Geurts S, Tilly MJ, et al. Heart rate variability is associated with left ventricular systolic, diastolic function and incident heart failure in the general population. BMC Med. 2022;20(1):91.
Arshi B, Chen J, Ikram MA, Zillikens MC, Kavousi M. Advanced glycation end-products, cardiac function and heart failure in the general population: The Rotterdam Study. Diabetologia. 2023;66(3):472-81.
Arshi B, Aliahmad HA, Ikram MA, Bos D, Kavousi M. Epicardial Fat Volume, Cardiac Function, and Incident Heart Failure: The Rotterdam Study. J Am Heart Assoc. 2023;12(1):e026197.
van der Toorn JE, Rueda-Ochoa OL, van der Schaft N, et al. Arterial calcification at multiple sites: sex-specific cardiovascular risk profiles and mortality risk-the Rotterdam Study. BMC Med. 2020;18(1):263.
van der Toorn JE, Bos D, Arshi B, et al. Arterial calcification at different sites and prediction of atherosclerotic cardiovascular disease among women and men. Atherosclerosis. 2021;337:27–34.
Kaiser Y, Singh SS, Zheng KH, et al. Lipoprotein(a) is robustly associated with aortic valve calcium. Heart. 2021;107(17):1422-8.
Kaiser Y, van der Toorn JE, Singh SS, et al. Lipoprotein(a) is associated with the onset but not the progression of aortic valve calcification. Eur Heart J. 2022;43(39):3960-7. doi:6649081 [pii] ehac377 [pii] https://doi.org/10.1093/eurheartj/ehac377
Bos D, Arshi B, van den Bouwhuijsen QJA, et al. Atherosclerotic Carotid Plaque Composition and Incident Stroke and Coronary Events. J Am Coll Cardiol. 2021;77(11):1426-35.
van der Toorn JE, Bos D, Ikram MK, et al. Carotid Plaque Composition and Prediction of Incident Atherosclerotic Cardiovascular Disease. Circ Cardiovasc Imaging. 2022;15(3):e013602.
Mujaj B, Bos D, Kavousi M, et al. Serum insulin levels are associated with vulnerable plaque components in the carotid artery: the Rotterdam Study. Eur J Endocrinol. 2020;182(3):343-50.
Zhu F, Arshi B, Ikram MA, De Knegt RJ, Kavousi M. Sex-specific normal values and determinants of infrarenal abdominal aortic diameter among non-aneurysmal elderly population. Sci Rep. 2021;11(1):17762.
Bons LR, Rueda-Ochoa OL, El Ghoul K, et al. Sex-specific distributions and determinants of thoracic aortic diameters in the elderly. Heart. 2020;106(2):133-9.
Thijssen CGE, Mutluer FO, van der Toorn JE, et al. Longitudinal changes of thoracic aortic diameters in the general population aged 55 years or older. Heart. 2022.
Portilla-Fernandez E, Klarin D, Hwang SJ, et al. Genetic and clinical determinants of abdominal aortic diameter: genome-wide association studies, exome array data and Mendelian randomization study. Hum Mol Genet. 2022;31(20):3566-79.
Rueda-Ochoa OL, Bons LR, Zhu F, et al. Thoracic Aortic Diameter and Cardiovascular Events and Mortality among Women and Men. Radiology. 2022;304(1):208-15.
Ahmadizar F, Wang K, Aribas E, et al. Impaired fasting glucose, type 2 diabetes mellitus, and lifetime risk of cardiovascular disease among women and men: the Rotterdam Study. BMJ Open Diabetes Res Care. 2021;9(1).
van Herpt TTW, Ligthart S, Leening MJG, et al. Lifetime risk to progress from pre-diabetes to type 2 diabetes among women and men: comparison between American Diabetes Association and World Health Organization diagnostic criteria. BMJ Open Diabetes Res Care. 2020;8(2).
Ligthart S, Hasbani NR, Ahmadizar F, et al. Genetic susceptibility, obesity and lifetime risk of type 2 diabetes: The ARIC study and Rotterdam Study. Diabet Med. 2021;38(10):e14639.
Wang K, Kavousi M, Voortman T, Ikram MA, Ghanbari M, Ahmadizar F. Cardiovascular health, genetic predisposition, and lifetime risk of type 2 diabetes. Eur J Prev Cardiol. 2022;28(16):1850-7.
Wu P, Moon JY, Daghlas I, et al. Obesity Partially Mediates the Diabetogenic Effect of Lowering LDL Cholesterol. Diabetes Care. 2022;45(1):232-40.
Brahimaj A, Ahmadizar F, Vernooij MW, et al. Epicardial fat volume and the risk of cardiometabolic diseases among women and men from the general population. Eur J Prev Cardiol. 2022;28(18):e14-e6.
Khan SR, Peeters RP, van Hagen PM, Dalm V, Chaker L. Determinants and Clinical Implications of Thyroid Peroxidase Antibodies in Middle-Aged and Elderly Individuals: The Rotterdam Study. Thyroid. 2022;32(1):78–89.
Fani L, Roa Dueñas O, Bos D, et al. Thyroid Status and Brain Circulation: The Rotterdam Study. J Clin Endocrinol Metab. 2022;107(3):e1293-e302.
Roa Dueñas OH, Koolhaas C, Voortman T, et al. Thyroid Function and Physical Activity: A Population-Based Cohort Study. Thyroid. 2021;31(6):870-5.
Syrogiannouli L, Wildisen L, Meuwese C, et al. Incorporating Baseline Outcome Data in Individual Participant Data Meta-Analysis of Non-randomized Studies. Front Psychiatry. 2022;13:774251. https://doi.org/10.3389/fpsyt.2022.774251
Zhou W, Brumpton B, Kabil O, et al. GWAS of thyroid stimulating hormone highlights pleiotropic effects and inverse association with thyroid cancer. Nat Commun. 2020;11(1):3981.
Xu Y, Derakhshan A, Hysaj O, et al. The optimal healthy ranges of thyroid function defined by the risk of cardiovascular disease and mortality: systematic review and individual participant data meta-analysis. Lancet Diabetes Endocrinol. 2023;11(10):743-54. doi:S2213-8587(23)00227-9 [pii] https://doi.org/10.1016/S2213-8587(23)00227-9
van der Burgh AC, Rizopoulos D, Ikram MA, Hoorn EJ, Chaker L. Determinants of the Evolution of Kidney Function With Age. Kidney Int Rep. 2021;6(12):3054-63.
van der Burgh AC, Stricker BH, Rizopoulos D, Ikram MA, Hoorn EJ, Chaker L. Kidney function and the risk of sudden cardiac death in the general population. Clin Kidney J. 2022;15(8):1524-33.
Nelson RG, Grams ME, Ballew SH, et al. Development of Risk Prediction Equations for Incident Chronic Kidney Disease. Jama. 2019;322(21):2104-14.
Gorski M, Jung B, Li Y, et al. Meta-analysis uncovers genome-wide significant variants for rapid kidney function decline. Kidney Int. 2021;99(4):926-39.
Schlosser P, Tin A, Matias-Garcia PR, et al. Meta-analyses identify DNA methylation associated with kidney function and damage. Nat Commun. 2021;12(1):7174.
Khan SR, Chaker L, Ikram MA, Peeters RP, van Hagen PM, Dalm V. Determinants and Reference Ranges of Serum Immunoglobulins in Middle-Aged and Elderly Individuals: a Population-Based Study. J Clin Immunol. 2021;41(8):1902-14.
Khan SR, Vanoverschelde A, Lahousse L, et al. Serum Immunoglobulins, Pneumonia Risk, and Lung Function in Middle-Aged and Older Individuals: A Population-Based Cohort Study. Front Immunol. 2022;13:868973.
Kemper CH, Peters PW. Migration and proliferation of primordial germ cells in the rat. Teratology. 1987;36(1):117-24. https://doi.org/10.1002/tera.1420360115
Khan SR, Yaqub A, Ikram MK, et al. The association of serum immunoglobulins with cognition and dementia: the Rotterdam Study. J Neurol. 2023;270(1):423-32.
Arinze JT, de Roos EW, Karimi L, Verhamme KMC, Stricker BH, Brusselle GG. Prevalence and incidence of, and risk factors for chronic cough in the adult population: the Rotterdam Study. ERJ Open Res. 2020;6(2).
Arinze JT, Verhamme KMC, Luik AI, Stricker B, van Meurs JBJ, Brusselle GG. The interrelatedness of chronic cough and chronic pain. Eur Respir J. 2021;57(5).
Arinze JT, Hofman A, de Roos EW, et al. The interrelationship of chronic cough and depression: a prospective population-based study. ERJ Open Res. 2022;8(2).
Benz E, Trajanoska K, Schoufour JD, et al. Sarcopenia in older people with chronic airway diseases: the Rotterdam study. ERJ Open Res. 2021;7(1).
Trajanoska K, Schoufour JD, Darweesh SK, et al. Sarcopenia and Its Clinical Correlates in the General Population: The Rotterdam Study. J Bone Miner Res. 2018;33(7):1209-18.
Wang AL, Lahousse L, Dahlin A, et al. Novel genetic variants associated with inhaled corticosteroid treatment response in older adults with asthma. Thorax. 2023;78(5):432-41.
Edris A, de Roos EW, McGeachie MJ, et al. Pharmacogenetics of inhaled corticosteroids and exacerbation risk in adults with asthma. Clin Exp Allergy. 2022;52(1):33–45.
Demenais F, Margaritte-Jeannin P, Barnes KC, et al. Multiancestry association study identifies new asthma risk loci that colocalize with immune-cell enhancer marks. Nat Genet. 2018;50(1):42–53.
Brusselle GG, Koppelman GH. Biologic Therapies for Severe Asthma. N Engl J Med. 2022;386(2):157-71. https://doi.org/10.1056/NEJMra2032506
Sarin KY, Lin Y, Daneshjou R, et al. Genome-wide meta-analysis identifies eight new susceptibility loci for cutaneous squamous cell carcinoma. Nat Commun. 2020;11(1):820.
Tokez S, Alblas M, Nijsten T, Pardo LM, Wakkee M. Predicting keratinocyte carcinoma in patients with actinic keratosis: development and internal validation of a multivariable risk-prediction model. Br J Dermatol. 2020;183(3):495–502.
George CD, Tokez S, Hollestein L, et al. Longitudinal Assessment of the Prevalence of Actinic Keratosis and Extensive Risk Factor Evaluation: An Update from the Rotterdam Study. J Invest Dermatol. 2023. doi:S0022-202X(23)02056-0 [pii] https://doi.org/10.1016/j.jid.2023.02.042
Allouche J, Rachmin I, Adhikari K, et al. NNT mediates redox-dependent pigmentation via a UVB- and MITF-independent mechanism. Cell. 2021;184(16):4268-83 e20.
Mekić S, Gunn DA, Jacobs LC, et al. Genetic Susceptibility to Dry Skin in a General Middle-Aged to Elderly Population: A GWAS. J Invest Dermatol. 2021;141(8):2077-9 e5.
Mekić S, Wigmann C, Gunn DA, et al. Genetics of facial telangiectasia in the Rotterdam Study: a genome-wide association study and candidate gene approach. J Eur Acad Dermatol Venereol. 2021;35(3):749-54.
Mekić S, Hamer MA, Wigmann C, et al. Epidemiology and determinants of facial telangiectasia: a cross-sectional study. J Eur Acad Dermatol Venereol. 2020;34(4):821-6.
Mekic S, Pardo LM, Gunn DA, et al. Younger facial looks are associate with a lower likelihood of several age-related morbidities in the middle-aged to elderly. Br J Dermatol. 2023;188(3):390-5. doi:6979846 [pii] https://doi.org/10.1093/bjd/ljac100
Sanders MGH, Nijsten T, Verlouw J, Kraaij R, Pardo LM. Composition of cutaneous bacterial microbiome in seborrheic dermatitis patients: A cross-sectional study. PLoS One. 2021;16(5):e0251136.
Ikram MA, Brusselle G, Ghanbari M, et al. Objectives, design and main findings until 2020 from the Rotterdam Study. Eur J Epidemiol. 2020;35(5):483–517.
Ikram MA, van der Lugt A, Niessen WJ, et al. The Rotterdam Scan Study: design update 2016 and main findings. Eur J Epidemiol. 2015;30(12):1299-315. doi:10.1007/s10654-015-0105-7 [pii] 105 [pii] https://doi.org/10.1007/s10654-015-0105-7
Hoogendam YY, Hofman A, van der Geest JN, van der Lugt A, Ikram MA. Patterns of cognitive function in aging: the Rotterdam Study. Eur J Epidemiol. 2014;29(2):133-40. https://doi.org/10.1007/s10654-014-9885-4
Verlinden VJ, van der Geest JN, Hoogendam YY, Hofman A, Breteler MM, Ikram MA. Gait patterns in a community-dwelling population aged 50 years and older. Gait Posture. 2013;37(4):500-5. doi:S0966-6362(12)00332-3 [pii] https://doi.org/10.1016/j.gaitpost.2012.09.005
Hanewinckel R, Drenthen J, van Oijen M, Hofman A, van Doorn PA, Ikram MA. Prevalence of polyneuropathy in the general middle-aged and elderly population. Neurology. 2016;87(18):1892-8. doi:WNL.0000000000003293 [pii] https://doi.org/10.1212/WNL.0000000000003293
Wolters FJ, Chibnik LB, Waziry R, et al. Twenty-seven-year time trends in dementia incidence in Europe and the United States: The Alzheimer Cohorts Consortium. Neurology. 2020;95(5):e519-e31. doi:WNL.0000000000010022 [pii] NEUROLOGY2019008235 [pii] https://doi.org/10.1212/WNL.0000000000010022
Dommershuijsen LJ, Heshmatollah A, Darweesh SKL, Koudstaal PJ, Ikram MA, Ikram MK. Life expectancy of parkinsonism patients in the general population. Parkinsonism Relat Disord. 2020;77:94-9. doi:S1353-8020(20)30195-4 [pii] https://doi.org/10.1016/j.parkreldis.2020.06.018
van der Willik KD, Licher S, Vinke EJ, et al. Trajectories of Cognitive and Motor Function Between Ages 45 and 90 Years: A Population-Based Study. J Gerontol A Biol Sci Med Sci. 2021;76(2):297–306. doi:5880609 [pii] glaa187 [pii] https://doi.org/10.1093/gerona/glaa187
Heshmatollah A, Dommershuijsen LJ, Fani L, Koudstaal PJ, Ikram MA, Ikram MK. Long-term trajectories of decline in cognition and daily functioning before and after stroke. J Neurol Neurosurg Psychiatry. 2021;92(11):1158-63. doi:jnnp-2021-326043 [pii] https://doi.org/10.1136/jnnp-2021-326043
Mooldijk SS, Yaqub A, Wolters FJ, et al. Life expectancy with and without dementia in persons with mild cognitive impairment in the community. J Am Geriatr Soc. 2022;70(2):481-9. doi:JGS17520 [pii] https://doi.org/10.1111/jgs.17520
Dommershuijsen LJ, Boon AJW, Ikram MK. Probing the Pre-diagnostic Phase of Parkinson’s Disease in Population-Based Studies. Front Neurol. 2021;12:702502. https://doi.org/10.3389/fneur.2021.702502
Dommershuijsen LJ, Heshmatollah A, Mattace Raso FUS, Koudstaal PJ, Ikram MA, Ikram MK. Orthostatic Hypotension: A Prodromal Marker of Parkinson’s Disease? Mov Disord. 2021;36(1):164-70. doi:MDS28303 [pii] https://doi.org/10.1002/mds.28303
Heshmatollah A, Fani L, Koudstaal PJ, Ghanbari M, Ikram MA, Ikram MK. Plasma beta-Amyloid, Total-Tau, and Neurofilament Light Chain Levels and the Risk of Stroke: A Prospective Population-Based Study. Neurology. 2022;98(17):e1729-e37. doi:WNL.0000000000200004 [pii] https://doi.org/10.1212/WNL.0000000000200004
Heshmatollah A, Ma Y, Fani L, Koudstaal PJ, Ikram MA, Ikram MK. Visit-to-visit blood pressure variability and the risk of stroke in the Netherlands: A population-based cohort study. PLoS Med. 2022;19(3):e1003942. doi:PMEDICINE-D-21-01404 [pii] https://doi.org/10.1371/journal.pmed.1003942
Holstege H, Hulsman M, Charbonnier C, et al. Exome sequencing identifies rare damaging variants in ATP8B4 and ABCA1 as risk factors for Alzheimer’s disease. Nat Genet. 2022;54(12):1786-94. doi:10.1038/s41588-022-01208-7 [pii] 1208 [pii] https://doi.org/10.1038/s41588-022-01208-7
Mishra A, Malik R, Hachiya T, et al. Stroke genetics informs drug discovery and risk prediction across ancestries. Nature. 2022;611(7934):115-23. doi:10.1038/s41586-022-05165-3 [pii] 5165 [pii] https://doi.org/10.1038/s41586-022-05165-3
Taams NE, Drenthen J, Hanewinckel R, Ikram MA, van Doorn PA. Prevalence and Risk Factor Profiles for Chronic Axonal Polyneuropathy in the General Population. Neurology. 2022. doi:WNL.0000000000201168 [pii] https://doi.org/10.1212/WNL.0000000000201168
van der Velpen IF, Vlasov V, Evans TE, et al. Subcortical brain structures and the risk of dementia in the Rotterdam Study. Alzheimers Dement. 2023;19(2):646-57.
Yaqub A, Mens MMJ, Klap JM, et al. Genome-wide profiling of circulatory microRNAs associated with cognition and dementia. Alzheimers Dement. 2023;19(4):1194-203.
Mooldijk SS, Dommershuijsen LJ, de Feijter M, Luik AI. Trajectories of depression and anxiety during the COVID-19 pandemic in a population-based sample of middle-aged and older adults. J Psychiatr Res. 2022;149:274-80.
de Feijter M, Kocevska D, Blanken TF, van der Velpen IF, Ikram MA, Luik AI. The network of psychosocial health in middle-aged and older adults during the first COVID-19 lockdown. Soc Psychiatry Psychiatr Epidemiol. 2022;57(12):2469-79.
Hofman A, Voortman T, Ikram MA, Luik AI. Substitutions of physical activity, sedentary behaviour and sleep: associations with mental health in middle-aged and elderly persons. J Epidemiol Community Health. 2022;76(2):175-81.
de Feijter M, Kocevska D, Ikram MA, Luik AI. The bidirectional association of 24-h activity rhythms and sleep with depressive symptoms in middle-aged and elderly persons. Psychol Med. 2023;53(4):1418-25.
Hofman A, Lier I, Ikram MA, van Wingerden M, Luik AI. Uncovering psychiatric phenotypes using unsupervised machine learning: A data-driven symptoms approach. Eur Psychiatry. 2023;66(1):e27.
de Feijter M, Katimertzoglou A, Tiemensma J, Ikram MA, Luik AI. Polysomnography-estimated sleep and the negative feedback loop of the hypothalamic-pituitary-adrenal (HPA) axis. Psychoneuroendocrinology. 2022;141:105749.
de Feijter M, Tiemensma J, Ikram MA, Stricker BH, Luik AI. The longitudinal association of sleep and 24-hour activity rhythms with cortisol response to a very low dose of dexamethasone. Sleep Health. 2022;8(4):398–405.
Özel F, Hilal S, de Feijter M, et al. Associations of neuroimaging markers with depressive symptoms over time in middle-aged and elderly persons. Psychol Med. 2023;53(10):4355-63.
van der Velpen IF, de Feijter M, Raina R, et al. Psychosocial health modifies associations between HPA-axis function and brain structure in older age. Psychoneuroendocrinology. 2023;153:106106.
Zijlmans JL, Vernooij MW, Ikram MA, Luik AI. The role of cognitive and brain reserve in late-life depressive events: The Rotterdam Study. J Affect Disord. 2023;320:211-7.
Zijlmans JL, Riemens MS, Vernooij MW, Ikram MA, Luik AI. Sleep, 24-Hour Activity Rhythms, and Cognitive Reserve: A Population-Based Study. J Alzheimers Dis. 2023;91(2):663-72.
Lysen TS, Luik AI, Ikram MK, Tiemeier H, Ikram MA. Actigraphy-estimated sleep and 24-hour activity rhythms and the risk of dementia. Alzheimers Dement. 2020;16(9):1259-67.
Lysen TS, Ikram MA, Ghanbari M, Luik AI. Sleep, 24-h activity rhythms, and plasma markers of neurodegenerative disease. Sci Rep. 2020;10(1):20691.
Lysen TS, Zonneveld HI, Muetzel RL, et al. Sleep and resting-state functional magnetic resonance imaging connectivity in middle-aged adults and the elderly: A population-based study. J Sleep Res. 2020;29(5):e12999.
Lysen TS, Yilmaz P, Dubost F, et al. Sleep and perivascular spaces in the middle-aged and elderly population. J Sleep Res. 2022;31(2):e13485.
Chen J, Waqas K, Tan RC, Voortman T, Ikram MA, Nijsten TE, De Groot LC, Uitterlinden AG, Zillikens MC. The association between dietary and skin advanced glycation end products: the Rotterdam Study. Am J Clin Nut. 2020;112(1):129–37. https://doi.org/10.1093/ajcn/nqaa117
Mormile R. Comment on Koromani et al. Vertebral Fractures in Individuals With Type 2 Diabetes: More Than Skeletal Complications Alone. Diabetes Care 2020;43:137–44. https://doi.org/10.2337/dc19-2460
Xiao T, Ghatan S, Mooldijk SS, et al. Association of Bone Mineral Density and Dementia: The Rotterdam Study. Neurology. 2023;100(20):e2125-e33.
Benz E, Trajanoska K, Schoufour JD, Lahousse L, de Roos EW, Terzikhan N, Medina-Gomez C, Verhamme K, Williams R, Stricker BH, Franco OH. Sarcopenia in older people with chronic airway diseases: the Rotterdam study. ERJ Open Res. 2021;7(1). https://doi.org/10.1183/23120541.00522-2020
Benz E, Wijnant SR, Trajanoska K, Arinze JT, de Roos EW, de Ridder M, Williams R, van Rooij F, Verhamme KM, Ikram MA, Stricker BH. Sarcopenia, systemic immune-inflammation index and all-cause mortality in middle-aged and older people with COPD and asthma: a population-based study. ERJ Open Res. 2022;8(1). https://doi.org/10.1183/23120541.00628-2021
Boer CG, Hatzikotoulas K, Southam L, et al. Deciphering osteoarthritis genetics across 826,690 individuals from 9 populations. Cell. 2021;184(18):4784-818 e17.
Boer CG, Szilagyi I, Nguyen NL, et al. Vitamin K antagonist anticoagulant usage is associated with increased incidence and progression of osteoarthritis. Ann Rheum Dis. 2021;80(5):598–604.
Loeser RF, Berenbaum F, Kloppenburg M. Vitamin K and osteoarthritis: is there a link? Ann Rheum Dis. 2021;80(5):547-9.
Sedaghati-Khayat B, Boer CG, Runhaar J, et al. Risk Assessment for Hip and Knee Osteoarthritis Using Polygenic Risk Scores. Arthritis Rheumatol. 2022;74(9):1488-96.
Medina-Gomez C, Mullin BH, Chesi A, et al. Bone mineral density loci specific to the skull portray potential pleiotropic effects on craniosynostosis. Commun Biol. 2023;6(1):691.
Szilagyi IA, Vallerga CL, Boer CG, et al. Plasma proteomics identifies CRTAC1 as a biomarker for osteoarthritis severity and progression. Rheumatology (Oxford). 2023;62(3):1286-95.
Boer CG, Radjabzadeh D, Medina-Gomez C, et al. Intestinal microbiome composition and its relation to joint pain and inflammation. Nat Commun. 2019;10(1):4881.
Waqas K, Chen J, Trajanoska K, Ikram MA, Uitterlinden AG, Rivadeneira F, Zillikens MC. Skin autofluorescence, a noninvasive biomarker for advanced glycation end-products, is associated with sarcopenia. J Clin Endocrinol Metabol. 2022;107(2):e793–803. https://doi.org/10.1210/clinem/dgab632
Waqas K, Chen J, Rivadeneira F, Uitterlinden AG, Voortman T, Zillikens MC. Skin autofluorescence, a noninvasive biomarker of advanced glycation end-products, is associated with frailty: the rotterdam study. J Gerontol: Series A. 2022;77(10):2032–9. https://doi.org/10.1093/gerona/glac025
Waqas K, Chen J, Koromani F, Trajanoska K, van der Eerden BC, Uitterlinden AG, Rivadeneira F, Zillikens MC. Skin autofluorescence, a noninvasive biomarker for advanced glycation end-products, is associated with prevalent vertebral and major osteoporotic fractures: the Rotterdam study. J Bone Min Res. 2020;35(10):1904–13. https://doi.org/10.1002/jbmr.4096
Kouyoumdjian P, Mansour J, Haignère V, Demattei C, Maury E, George D, Coulomb R. Hip-Spine relationship between sagittal balance of the Lumbo-Pelvi-Femoral complex and hip extension capacity: an EOS evaluation in a healthy caucasian population. Global Spine J. 2022:21925682221103831. https://doi.org/10.1177/21925682221103831
Deal JA, Betz J, Yaffe K, et al. Hearing Impairment and Incident Dementia and Cognitive Decline in Older Adults: The Health ABC Study. J Gerontol A Biol Sci Med Sci. 2017;72(5):703-9. doi:glw069 [pii] https://doi.org/10.1093/gerona/glw069
Livingston G, Sommerlad A, Orgeta V, et al. Dementia prevention, intervention, and care. Lancet. 2017;390(10113):2673-734. doi:S0140-6736(17)31363-6 [pii] https://doi.org/10.1016/S0140-6736(17)31363-6
Smits C, Kapteyn TS, Houtgast T. Development and validation of an automatic speech-in-noise screening test by telephone. Int J Audiol. 2004;43(1):15–28. https://doi.org/10.1080/14992020400050004
Cox RM, Alexander GC. The International Outcome Inventory for Hearing Aids (IOI-HA): psychometric properties of the English version. Int J Audiol. 2002;41(1):30-5. https://doi.org/10.3109/14992020209101309
Newman CW, Sandridge SA, Bolek L. Development and psychometric adequacy of the screening version of the tinnitus handicap inventory. Otol Neurotol. 2008;29(3):276-81. https://doi.org/10.1097/MAO.0b013e31816569c4
Trpchevska N, Freidin MB, Broer L, et al. Genome-wide association meta-analysis identifies 48 risk variants and highlights the role of the stria vascularis in hearing loss. Am J Hum Genet. 2022;109(6):1077-91. doi:S0002-9297(22)00158-6 [pii] https://doi.org/10.1016/j.ajhg.2022.04.010
Croll PH, Vinke EJ, Armstrong NM, et al. Hearing loss and cognitive decline in the general population: a prospective cohort study. J Neurol. 2021;268(3):860-71. doi:10.1007/s00415-020-10208-8 [pii] 10208 [pii] https://doi.org/10.1007/s00415-020-10208-8
Oosterloo BC, Croll PH, Baatenburg de Jong RJ, Ikram MK, Goedegebure A. Prevalence of Tinnitus in an Aging Population and Its Relation to Age and Hearing Loss. Otolaryngol Head Neck Surg. 2021;164(4):859-68. doi:10.1177_0194599820957296 [pii] https://doi.org/10.1177/0194599820957296
Oosterloo BC, de Feijter M, Croll PH, Baatenburg de Jong RJ, Luik AI, Goedegebure A. Cross-sectional and Longitudinal Associations Between Tinnitus and Mental Health in a Population-Based Sample of Middle-aged and Elderly Persons. JAMA Otolaryngol Head Neck Surg. 2021;147(8):708-16. doi:2781095 [pii] ooi210022 [pii] https://doi.org/10.1001/jamaoto.2021.1049
Talwalkar JA, Kurtz DM, Schoenleber SJ, West CP, Montori VM. Ultrasound-based transient elastography for the detection of hepatic fibrosis: systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2007;5(10):1214-20. doi:S1542-3565(07)00753-7 [pii] https://doi.org/10.1016/j.cgh.2007.07.020
van Kleef LA, Ayada I, Alferink LJM, Pan Q, de Knegt RJ. Metabolic dysfunction-associated fatty liver disease improves detection of high liver stiffness: The Rotterdam Study. Hepatology. 2022;75(2):419-29. doi:01515467-202202000-00018 [pii] HEP32131 [pii] https://doi.org/10.1002/hep.32131
Ayada I, van Kleef LA, Alferink LJM, Li P, de Knegt RJ, Pan Q. Systematically comparing epidemiological and clinical features of MAFLD and NAFLD by meta-analysis: Focusing on the non-overlap groups. Liver Int. 2022;42(2):277-87. https://doi.org/10.1111/liv.15139
Xiao T, van Kleef LA, Ikram MK, de Knegt RJ, Ikram MA. Association of Nonalcoholic Fatty Liver Disease and Fibrosis With Incident Dementia and Cognition: The Rotterdam Study. Neurology. 2022;99(6):e565-e73. doi:WNL.0000000000200770 [pii] WNL-2022-200754 [pii] https://doi.org/10.1212/WNL.0000000000200770
van Kleef LA, Xiao T, Ikram MA, Ikram MK, de Knegt RJ. Sex-stratified associations between fatty liver disease and Parkinson’s disease: The Rotterdam study. Parkinsonism Relat Disord. 2023;106:105233. doi:S1353-8020(22)00402-3 [pii] https://doi.org/10.1016/j.parkreldis.2022.105233
van Kleef LA, Sonneveld MJ, Kavousi M, Ikram MA, de Man RA, de Knegt RJ. Fatty liver disease is not associated with increased mortality in the elderly: A prospective cohort study. Hepatology. 2023;77(2):585-93. doi:01515467-202302000-00024 [pii] https://doi.org/10.1002/hep.32635
van Kleef LA, Sonneveld MJ, Zhu F, Ikram MA, Kavousi M, de Knegt RJ. Liver stiffness is associated with excess mortality in the general population driven by heart failure: The Rotterdam Study. Liver Int. 2023;43(5):1000-7. https://doi.org/10.1111/liv.15538
Warnert EAH, Steketee RME, Vernooij MW, et al. Implementation and validation of ASL perfusion measurements for population imaging. Magn Reson Med. 2020;84(4):2048-54.
van Arendonk J, Neitzel J, Steketee RME, et al. Diabetes and hypertension are related to amyloid-beta burden in the population-based Rotterdam Study. Brain. 2023;146(1):337-48.
van den Brink H, Kopczak A, Arts T, et al. Zooming in on cerebral small vessel function in small vessel diseases with 7T MRI: Rationale and design of the “ZOOM@SVDs” study. Cereb Circ Cogn Behav. 2021;2:100013.
Dubost F, Adams H, Bortsova G, et al. 3D regression neural network for the quantification of enlarged perivascular spaces in brain MRI. Med Image Anal. 2019;51:89–100.
Evans TE, Knol MJ, Schwingenschuh P, et al. Determinants of Perivascular Spaces in the General Population: A Pooled Cohort Analysis of Individual Participant Data. Neurology. 2023;100(2):e107-e22.
Vinke EJ, de Groot M, Venkatraghavan V, et al. Trajectories of imaging markers in brain aging: the Rotterdam Study. Neurobiol Aging. 2018;71:32–40.
Vinke EJ, Huizinga W, Bergtholdt M, et al. Normative brain volumetry derived from different reference populations: impact on single-subject diagnostic assessment in dementia. Neurobiol Aging. 2019;84:9–16.
Lamballais S, Vinke EJ, Vernooij MW, Ikram MA, Muetzel RL. Cortical gyrification in relation to age and cognition in older adults. Neuroimage. 2020;212:116637.
Venkatraghavan V, Vinke EJ, Bron EE, et al. Progression along data-driven disease timelines is predictive of Alzheimer’s disease in a population-based cohort. Neuroimage. 2021;238:118233.
Ma Y, Song A, Viswanathan A, et al. Blood Pressure Variability and Cerebral Small Vessel Disease: A Systematic Review and Meta-Analysis of Population-Based Cohorts. Stroke. 2020;51(1):82-9. https://doi.org/10.1161/STROKEAHA.119.026739
Ma Y, Yilmaz P, Bos D, et al. Blood Pressure Variation and Subclinical Brain Disease. J Am Coll Cardiol. 2020;75(19):2387-99.
Cremers LGM, Wolters FJ, de Groot M, et al. Structural disconnectivity and the risk of dementia in the general population. Neurology. 2020;95(11):e1528-e37. doi:WNL.0000000000010231 [pii] https://doi.org/10.1212/WNL.0000000000010231
van den Beukel TC, van der Toorn JE, Vernooij MW, et al. Morphological Subtypes of Intracranial Internal Carotid Artery Arteriosclerosis and the Risk of Stroke. Stroke. 2022;53(4):1339-47.
Fani L, van Dam-Nolen DHK, Vernooij M, Kavousi M, van der Lugt A, Bos D. Circulatory markers of immunity and carotid atherosclerotic plaque. Atherosclerosis. 2021;325:69–74.
Kuiper LM, Ikram MK, Kavousi M, Vernooij MW, Ikram MA, Bos D. C-factor: a summary measure for systemic arterial calcifications. BMC Cardiovasc Disord. 2021;21(1):317.
Meister I, Zhang P, Sinha A, et al. High-Precision Automated Workflow for Urinary Untargeted Metabolomic Epidemiology. Anal Chem. 2021;93(12):5248-58.
Võsa U, Claringbould A, Westra HJ, et al. Large-scale cis- and trans-eQTL analyses identify thousands of genetic loci and polygenic scores that regulate blood gene expression. Nat Genet. 2021;53(9):1300-10.
Min JL, Hemani G, Hannon E, et al. Genomic and phenotypic insights from an atlas of genetic effects on DNA methylation. Nat Genet. 2021;53(9):1311-21.
Karabegović I, Portilla-Fernandez E, Li Y, et al. Epigenome-wide association meta-analysis of DNA methylation with coffee and tea consumption. Nat Commun. 2021;12(1):2830.
Portilla-Fernández E, Hwang SJ, Wilson R, et al. Meta-analysis of epigenome-wide association studies of carotid intima-media thickness. Eur J Epidemiol. 2021;36(11):1143-55.
Ochoa-Rosales C, Portilla-Fernandez E, Nano J, et al. Epigenetic Link Between Statin Therapy and Type 2 Diabetes. Diabetes Care. 2020;43(4):875-84.
Maas SCE, Vidaki A, Teumer A, et al. Validating biomarkers and models for epigenetic inference of alcohol consumption from blood. Clin Epigenetics. 2021;13(1):198.
Juvinao-Quintero DL, Marioni RE, Ochoa-Rosales C, et al. DNA methylation of blood cells is associated with prevalent type 2 diabetes in a meta-analysis of four European cohorts. Clin Epigenetics. 2021;13(1):40.
Maas SCE, Mens MMJ, Kühnel B, et al. Smoking-related changes in DNA methylation and gene expression are associated with cardio-metabolic traits. Clin Epigenetics. 2020;12(1):157.
Mens MMJ, Heshmatollah A, Fani L, Ikram MA, Ikram MK, Ghanbari M. Circulatory MicroRNAs as Potential Biomarkers for Stroke Risk: The Rotterdam Study. Stroke. 2021;52(3):945-53.
Zhang X, Mens MMJ, Abozaid YJ, et al. Circulatory microRNAs as potential biomarkers for fatty liver disease: the Rotterdam study. Aliment Pharmacol Ther. 2021;53(3):432-42.
Mens MMJ, Maas SCE, Klap J, et al. Multi-Omics Analysis Reveals MicroRNAs Associated With Cardiometabolic Traits. Front Genet. 2020;11:110.
Geurts S, Mens MMJ, Bos MM, Ikram MA, Ghanbari M, Kavousi M. Circulatory MicroRNAs in Plasma and Atrial Fibrillation in the General Population: The Rotterdam Study. Genes (Basel). 2021;13(1).
Abozaid YJ, Zhang X, Mens MMJ, et al. Plasma circulating microRNAs associated with obesity, body fat distribution, and fat mass: the Rotterdam Study. Int J Obes (Lond). 2022;46(12):2137-44.
Abozaid YJ, Ayada I, van Kleef LA, et al. Plasma proteomic signature of fatty liver disease: The Rotterdam Study. Hepatology. 2023;78(1):284-94.
Kurilshikov A, Medina-Gomez C, Bacigalupe R, et al. Large-scale association analyses identify host factors influencing human gut microbiome composition. Nat Genet. 2021;53(2):156-65.
Radjabzadeh D, Bosch JA, Uitterlinden AG, et al. Gut microbiome-wide association study of depressive symptoms. Nat Commun. 2022;13(1):7128.
Lakeman IMM, Rodríguez-Girondo M, Lee A, et al. Validation of the BOADICEA model and a 313-variant polygenic risk score for breast cancer risk prediction in a Dutch prospective cohort. Genet Med. 2020;22(11):1803-11.
Voortman T, Kiefte-de Jong JC, Ikram MA, et al. Adherence to the 2015 Dutch dietary guidelines and risk of non-communicable diseases and mortality in the Rotterdam Study. Eur J Epidemiol. 2017;32(11):993–1005.
Voortman T, Chen Z, Girschik C, Kavousi M, Franco OH, Braun KVE. Associations between macronutrient intake and coronary heart disease (CHD): The Rotterdam Study. Clin Nutr. 2021;40(11):5494-9.
de Crom TOE, Blekkenhorst L, Vernooij MW, Ikram MK, Voortman T, Ikram MA. Dietary nitrate intake in relation to the risk of dementia and imaging markers of vascular brain health: a population-based study. Am J Clin Nutr. 2023;118(2):352-9.
van der Schaft N, Trajanoska K, Rivadeneira F, Ikram MA, Schoufour JD, Voortman T. Total Dietary Antioxidant Capacity and Longitudinal Trajectories of Body Composition. Antioxidants (Basel). 2020;9(8).
Strikwerda AJ, Dommershuijsen LJ, Ikram MK, Voortman T. Diet Quality and Risk of Parkinson’s Disease: The Rotterdam Study. Nutrients. 2021;13(11).
de Crom TOE, Mooldijk SS, Ikram MK, Ikram MA, Voortman T. MIND diet and the risk of dementia: a population-based study. Alzheimers Res Ther. 2022;14(1):8.
Croll PH, Boelens M, Vernooij MW, et al. Associations of vitamin D deficiency with MRI markers of brain health in a community sample. Clin Nutr. 2021;40(1):72-8.
Koolhaas CM, van Rooij FJ, Cepeda M, Tiemeier H, Franco OH, Schoufour JD. Physical activity derived from questionnaires and wrist-worn accelerometers: comparability and the role of demographic, lifestyle, and health factors among a population-based sample of older adults. Clin Epidemiol. 2018;10:1–16.
Ronkainen J, Nedelec R, Atehortua A, et al. LongITools: Dynamic longitudinal exposome trajectories in cardiovascular and metabolic noncommunicable diseases. Environ Epidemiol. 2022;6(1):e184.
Ochoa-Rosales C, van der Schaft N, Braun KVE, et al. C-reactive protein partially mediates the inverse association between coffee consumption and risk of type 2 diabetes: The UK Biobank and the Rotterdam study cohorts. Clin Nutr. 2023;42(5):661-9.
van Westing AC, Ochoa-Rosales C, van der Burgh AC, et al. Association of habitual coffee consumption and kidney function: A prospective analysis in the Rotterdam Study. Clin Nutr. 2023;42(2):83–92.
Slurink IAL, Voortman T, Ochoa-Rosales C, et al. Dairy Product Consumption in Relation to Incident Prediabetes and Longitudinal Insulin Resistance in the Rotterdam Study. Nutrients. 2022;14(3).
Jacobo Cejudo MG, Ochoa-Rosales C, Ahmadizar F, Kavousi M, Geleijnse JM, Voortman T. The healthy beverage index is not associated with insulin resistance, prediabetes and type 2 diabetes risk in the Rotterdam Study. Eur J Nutr. 2023;62(7):3021-31.
Verhoog S, Braun KVE, Bano A, et al. Associations of Activity and Sleep With Quality of Life: A Compositional Data Analysis. Am J Prev Med. 2020;59(3):412-9.
Galle SA, Liu J, Bonnechère B, et al. The long-term relation between physical activity and executive function in the Rotterdam Study. Eur J Epidemiol. 2023;38(1):71–81.
Hofman A, Rodriguez-Ayllon M, Vernooij MW, et al. Physical activity levels and brain structure in middle-aged and older adults: a bidirectional longitudinal population-based study. Neurobiol Aging. 2023;121:28–37.
Wang Z, Emmerich A, Pillon NJ, et al. Genome-wide association analyses of physical activity and sedentary behavior provide insights into underlying mechanisms and roles in disease prevention. Nat Genet. 2022;54(9):1332–44.
Mooldijk SS, de Crom TOE, Ikram MK, Ikram MA, Voortman T. Adiposity in the older population and the risk of dementia: The Rotterdam Study. Alzheimers Dement. 2023;19(5):2047–55.
de Crom TOE, Ginos BNR, Oudin A, Ikram MK, Voortman T, Ikram MA. Air Pollution and the Risk of Dementia: The Rotterdam Study. J Alzheimers Dis. 2023;91(2):603–13.
Chen Z, Radjabzadeh D, Chen L, et al. Association of Insulin Resistance and Type 2 Diabetes With Gut Microbial Diversity: A Microbiome-Wide Analysis From Population Studies. JAMA Netw Open. 2021;4(7):e2118811.
Chen J, Radjabzadeh D, Medina-Gomez C, et al. Advanced Glycation End Products (AGEs) in Diet and Skin in Relation to Stool Microbiota: The Rotterdam Study. Nutrients. 2023;15(11).
Karabegović I, Abozaid Y, Maas SCE, et al. Plasma MicroRNA Signature of Alcohol Consumption: The Rotterdam Study. J Nutr. 2023;152(12):2677–88.
Karabegović I, Maas SCE, Shuai Y, et al. Smoking-related dysregulation of plasma circulating microRNAs: the Rotterdam study. Hum Genomics. 2023;17(1):61.
Funding
Since 2018 the Rotterdam Study has been designated a Core Facility by the Erasmus MC. As Core Facility the Rotterdam Study receives infrastructural funding directly from the institute that covers a part of its basic funding. The remaining infrastructural costs are covered by the participating departments and institutes. External funding is acquired primarily to fund ongoing and well-delineated scientific projects within the Rotterdam Study and is mainly obtained on project-basis. Important external fundings bodies include – but are certainly not limited to – Netherlands Research Organization (NWO), Netherlands Organization for Health Research and Development (ZonMw), European Commission (FP6, FP7, Horizon2020, ERC), and National Institute of Health (NIH).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ikram, M.A., Kieboom, B.C., Brouwer, W.P. et al. The Rotterdam Study. Design update and major findings between 2020 and 2024. Eur J Epidemiol 39, 183–206 (2024). https://doi.org/10.1007/s10654-023-01094-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10654-023-01094-1
Keywords
- Biomarkers
- Cancer and related diseases
- Cardiovascular diseases
- Cohort study
- Dermatological diseases
- Endocrine diseases
- Epidemiologic methods
- Genetic and molecular epidemiology
- Nutrition and lifestyle epidemiology
- Liver diseases
- Neurological diseases
- Oncology
- Ophthalmic diseases
- Otolaryngological diseases
- Pharmacoepidemiology
- Population imaging
- Renal diseases
- Psychiatric diseases
- Respiratory diseases
- Responsible research
- Risk factors