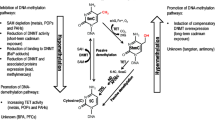
Abstract
Today, the rapid development of science and technology and the rapid change in economy and society are changing the way of life of human beings and affecting the natural, living, working, and internal environment on which human beings depend. At the same time, the global incidence of cancer has increased significantly yearly, and cancer has become the number one killer that threatens human health. Studies have shown that diet, living habits, residential environment, mental and psychological factors, intestinal flora, genetics, social factors, and viral and non-viral infections are closely related to human cancer. However, the molecular mechanisms of the environment and cancer development remain to be further explored. In recent years, DNA methylation has become a key hub and bridge for environmental and cancer research. Some environmental factors can alter the hyper/hypomethylation of human cancer suppressor gene promoters, proto-oncogene promoters, and the whole genome, causing low/high expression or gene mutation of related genes, thereby exerting oncogenic or anticancer effects. It is expected to develop early warning markers of cancer environment based on DNA methylation, thereby providing new methods for early detection of cancers, diagnosis, and targeted therapy. This review systematically expounds on the internal mechanism of environmental factors affecting cancer by changing DNA methylation, aiming to help establish the concept of cancer prevention and improve people's health.









Similar content being viewed by others
Reference
Cavalli, G., & Heard, E. (2019). Advances in epigenetics link genetics to the environment and disease. Nature, 571(7766), 489–499. https://doi.org/10.1038/s41586-019-1411-0. Epub 20190724.
Taby, R., & Issa, J. P. (2010). Cancer epigenetics. CA: A Cancer Journal for Clinicians, 60(6), 376–392. https://doi.org/10.3322/caac.20085. Epub 20101019.
Esteller, M. (2008). Epigenetics in cancer. New England Journal of Medicine, 358(11), 1148–1159. https://doi.org/10.1056/NEJMra072067
Strahl, B. D., & Allis, C. D. (2000). The language of covalent histone modifications. Nature, 403(6765), 41–45. https://doi.org/10.1038/47412
He, L., & Hannon, G. J. (2004). Micrornas: Small Rnas with a big role in gene regulation. Nature Reviews Genetics, 5(7), 522–531. https://doi.org/10.1038/nrg1379
Lapeyre, J. N., & Becker, F. F. (1979). 5-Methylcytosine content of nuclear DNA during chemical hepatocarcinogenesis and in carcinomas which result. Biochemical and Biophysical Research Communications, 87(3), 698–705. https://doi.org/10.1016/0006-291x(79)92015-1
Issa, J. P. (2008). Cancer prevention: Epigenetics steps up to the plate. Cancer Prevention Research (Phila), 1(4), 219–222. https://doi.org/10.1158/1940-6207.Capr-08-0029. Epub 20080319.
Michalak, E. M., Burr, M. L., Bannister, A. J., & Dawson, M. A. (2019). The roles of DNA, Rna and histone methylation in ageing and cancer. Nature Reviews Molecular Cell Biology, 20(10), 573–589. https://doi.org/10.1038/s41580-019-0143-1. Epub 20190703.
Henikoff, S., & Shilatifard, A. (2011). Histone modification: Cause or cog? Trends in Genetics, 27(10), 389–396. https://doi.org/10.1016/j.tig.2011.06.006. Epub 20110720.
Barbieri, I., Tzelepis, K., Pandolfini, L., Shi, J., Millán-Zambrano, G., Robson, S. C., et al. (2017). Promoter-Bound Mettl3 maintains myeloid leukaemia by M(6)a-dependent translation control. Nature, 552(7683), 126–131. https://doi.org/10.1038/nature24678. Epub 20171127.
Feil, R., & Fraga, M. F. (2012). Epigenetics and the environment: Emerging patterns and implications. Nature Reviews Genetics, 13(2), 97–109. https://doi.org/10.1038/nrg3142. Epub 20120104.
Ongenaert, M., Van Neste, L., De Meyer, T., Menschaert, G., Bekaert, S., & Van Criekinge, W. (2008). Pubmeth: A cancer methylation database combining text-mining and expert annotation. Nucleic acids research, 36, D842–D846. https://doi.org/10.1093/nar/gkm788. Epub 20071011.
Curtin, N. J. (2012). DNA repair dysregulation from cancer driver to therapeutic target. Nature Reviews Cancer, 12(12), 801–817. https://doi.org/10.1038/nrc3399
Robertson, K. D., Ait-Si-Ali, S., Yokochi, T., Wade, P. A., Jones, P. L., & Wolffe, A. P. (2000). Dnmt1 forms a complex with Rb, E2f1 and Hdac1 and represses transcription from E2f-responsive promoters. Nature Genetics, 25(3), 338–342. https://doi.org/10.1038/77124
Zhang, Y., Yang, L., Kucherlapati, M., Hadjipanayis, A., Pantazi, A., Bristow, C. A., et al. (2019). Global impact of somatic structural variation on the DNA methylome of human cancers. Genome Biology, 20(1), 209. https://doi.org/10.1186/s13059-019-1818-9. Epub 20191015.
Daura-Oller, E., Cabre, M., Montero, M. A., Paternain, J. L., & Romeu, A. (2009). Specific gene hypomethylation and cancer: New insights into coding region feature trends. Bioinformation, 3(8), 340–343. https://doi.org/10.6026/97320630003340. Epub 20090421.
Howard, G., Eiges, R., Gaudet, F., Jaenisch, R., & Eden, A. (2008). Activation and transposition of endogenous retroviral elements in hypomethylation induced tumors in mice. Oncogene, 27(3), 404–408. https://doi.org/10.1038/sj.onc.1210631. Epub 20070709.
Yoder, J. A., Walsh, C. P., & Bestor, T. H. (1997). Cytosine methylation and the ecology of intragenomic parasites. Trends in Genetics, 13(8), 335–340. https://doi.org/10.1016/s0168-9525(97)01181-5
Laird, P. W. (2003). The power and the promise of DNA methylation markers. Nature Reviews Cancer, 3(4), 253–266. https://doi.org/10.1038/nrc1045
Tomasetti, C., Li, L., & Vogelstein, B. (2017). Stem cell divisions, somatic mutations, cancer etiology, and cancer prevention. Science, 355(6331), 1330–1334. https://doi.org/10.1126/science.aaf9011
Wu, S., Zhu, W., Thompson, P., & Hannun, Y. A. (2018). Evaluating intrinsic and non-intrinsic cancer risk factors. Nature Communications, 9(1), 3490. https://doi.org/10.1038/s41467-018-05467-z. Epub 20180828.
Colditz, G. A., & Wei, E. K. (2012). Preventability of cancer: The relative contributions of biologic and social and physical environmental determinants of cancer mortality. Annual Review of Public Health, 33, 137–156. https://doi.org/10.1146/annurev-publhealth-031811-124627. Epub 20120103.
Wu, S., Powers, S., Zhu, W., & Hannun, Y. A. (2016). Substantial contribution of extrinsic risk factors to cancer development. Nature, 529(7584), 43–47. https://doi.org/10.1038/nature16166. Epub 20151216.
Steel, N., Ford, J. A., Newton, J. N., Davis, A. C. J., Vos, T., Naghavi, M., et al. (2018). Changes in health in the countries of the UK and 150 english local authority areas 1990–2016: A systematic analysis for the global burden of disease study 2016. Lancet, 392(10158), 1647–1661. https://doi.org/10.1016/s0140-6736(18)32207-4. Epub 20181024.
Hecht, S. S. (2003). Tobacco carcinogens, their biomarkers and tobacco-induced cancer. Nature Reviews Cancer, 3(10), 733–744. https://doi.org/10.1038/nrc1190
Breitling, L. P., Yang, R., Korn, B., Burwinkel, B., & Brenner, H. (2011). Tobacco-smoking-related differential DNA methylation: 27k discovery and replication. The American Journal of Human Genetics, 88(4), 450–457. https://doi.org/10.1016/j.ajhg.2011.03.003. Epub 20110331.
Suter, M., Abramovici, A., Showalter, L., Hu, M., Shope, C. D., Varner, M., et al. (2010). In utero tobacco exposure epigenetically modifies placental Cyp1a1 expression. Metabolism, 59(10), 1481–1490. https://doi.org/10.1016/j.metabol.2010.01.013. Epub 20100511.
Di, Y. P., Zhao, J., & Harper, R. (2012). Cigarette smoke induces Muc5ac protein expression through the activation of Sp1. Journal of Biological Chemistry, 287(33), 27948–27958. https://doi.org/10.1074/jbc.M111.334375. Epub 20120614.
Liu, Q., Liu, L., Zhao, Y., Zhang, J., Wang, D., Chen, J., He, Y., Wu, J., Zhang, Z., & Liu, Z. (2011). Hypoxia induces genomic DNA demethylation through the activation of Hif-1α and transcriptional upregulation of Mat2a in hepatoma cells. Molecular Cancer Therapeutics, 10(6), 1113–1123. https://doi.org/10.1158/1535-7163.Mct-10-1010. Epub 20110401.
Battram, T., Richmond, R. C., Baglietto, L., Haycock, P. C., Perduca, V., Bojesen, S. E., et al. (2019). Appraising the causal relevance of DNA methylation for risk of lung cancer. International Journal of Epidemiology, 48(5), 1493–1504. https://doi.org/10.1093/ije/dyz190
Kim, D. H., Nelson, H. H., Wiencke, J. K., Zheng, S., Christiani, D. C., Wain, J. C., et al. (2001). P16(Ink4a) and histology-specific methylation of Cpg islands by exposure to tobacco smoke in non-small cell lung cancer. Cancer Research, 61(8), 3419–3424.
Zhang, R., Lai, L., Dong, X., He, J., You, D., Chen, C., Lin, L., Zhu, Y., Huang, H., Shen, S., & Wei, L. (2019). SIPA1L3 methylation modifies the benefit of smoking cessation on lung adenocarcinoma survival: an epigenomic–smoking interaction analysis. Molecular Oncology, 13(5), 1235–1248. https://doi.org/10.1002/1878-0261.12482. Epub 20190417.
Kaz, A. M., Wong, C. J., Luo, Y., Virgin, J. B., Washington, M. K., Willis, J. E., et al. (2011). DNA methylation profiling in barrett’s esophagus and esophageal adenocarcinoma reveals unique methylation signatures and molecular subclasses. Epigenetics, 6(12), 1403–1412. https://doi.org/10.4161/epi.6.12.18199
Wolff, E. M., Liang, G., Cortez, C. C., Tsai, Y. C., Castelao, J. E., Cortessis, V. K., et al. (2008). Runx3 methylation reveals that bladder tumors are older in patients with a history of smoking. Cancer Research, 68(15), 6208–6214. https://doi.org/10.1158/0008-5472.Can-07-6616
Cash, H. L., Tao, L., Yuan, J. M., Marsit, C. J., Houseman, E. A., Xiang, Y. B., Gao, Y. T., Nelson, H. H., & Kelsey, K. T. (2012). Line-1 hypomethylation is associated with bladder cancer risk among nonsmoking chinese. International Journal of Cancer, 130(5), 1151–1159. https://doi.org/10.1002/ijc.26098. Epub 20110525.
Wilhelm, C. S., Kelsey, K. T., Butler, R., Plaza, S., Gagne, L., Zens, M. S., Andrew, A. S., Morris, S., Nelson, H. H., Schned, A. R., & Karagas, M. R. (2010). Implications of Line1 methylation for bladder cancer risk in women. Clinical cancer research, 16(5), 1682–1689. https://doi.org/10.1158/1078-0432.Ccr-09-2983. Epub 20100223.
Wu, H. C., Wang, Q., Yang, H. I., Tsai, W. Y., Chen, C. J., & Santella, R. M. (2012). Global DNA methylation levels in white blood cells as a biomarker for hepatocellular carcinoma risk: A nested case-control study. Carcinogenesis, 33(7), 1340–1345. https://doi.org/10.1093/carcin/bgs160. Epub 20120512.
Shimazu, T., Asada, K., Charvat, H., Kusano, C., Otake, Y., Kakugawa, Y., et al. (2015). Association of gastric cancer risk factors with DNA methylation levels in gastric mucosa of healthy Japanese: A cross-sectional study. Carcinogenesis, 36(11), 1291–1298. https://doi.org/10.1093/carcin/bgv125. Epub 20150908.
Momi, N., Kaur, S., Ponnusamy, M. P., Kumar, S., Wittel, U. A., & Batra, S. K. (2012). Interplay between smoking-induced genotoxicity and altered signaling in pancreatic carcinogenesis. Carcinogenesis, 33(9), 1617–1628. https://doi.org/10.1093/carcin/bgs186. Epub 20120523.
Samadder, N. J., Vierkant, R. A., Tillmans, L. S., Wang, A. H., Lynch, C. F., Anderson, K. E., French, A. J., Haile, R. W., Harnack, L. J., Potter, J. D., & Slager, S. L. (2012). Cigarette smoking and colorectal cancer risk by kras mutation status among older women. The American journal of gastroenterology, 107(5), 782–789. https://doi.org/10.1038/ajg.2012.21. Epub 20120221.
Barrow, T. M., Klett, H., Toth, R., Böhm, J., Gigic, B., Habermann, N., Scherer, D., Schrotz-King, P., Skender, S., Abbenhardt-Martin, C., & Zielske, L. (2017). Smoking is associated with hypermethylation of the Apc 1a promoter in colorectal cancer: The colocare study. The Journal of Pathology, 243(3), 366–375. https://doi.org/10.1002/path.4955. Epub 20170929.
Enokida, H., Shiina, H., Urakami, S., Terashima, M., Ogishima, T., Li, L. C., et al. (2006). Smoking influences aberrant Cpg hypermethylation of multiple genes in human prostate carcinoma. Cancer, 106(1), 79–86. https://doi.org/10.1002/cncr.21577
Shui, I. M., Wong, C. J., Zhao, S., Kolb, S., Ebot, E. M., Geybels, M. S., et al. (2016). Prostate tumor DNA methylation is associated with cigarette smoking and adverse prostate cancer outcomes. Cancer, 122(14), 2168–2177. https://doi.org/10.1002/cncr.30045. Epub 20160503.
Lea, J. S., Coleman, R., Kurien, A., Schorge, J. O., Miller, D. S., Minna, J. D., et al. (2004). Aberrant P16 methylation is a biomarker for tobacco exposure in cervical squamous cell carcinogenesis. American Journal of Obstetrics and Gynecology, 190(3), 674–679. https://doi.org/10.1016/j.ajog.2003.09.036
Mayne, S. T., Playdon, M. C., & Rock, C. L. (2016). Diet, nutrition, and cancer: past, present and future. Nature Reviews Clinical Oncology, 13(8), 504–515. https://doi.org/10.1038/nrclinonc.2016.24. Epub 20160308.
Doll, R., & Peto, R. (1981). The causes of cancer: quantitative estimates of avoidable risks of cancer in the United States today. Journal of the National Cancer Institute, 66(6), 1191–1308.
World Health Organization. Who Report on Cancer: Setting Priorities, Investing Wisely and Providing Care for All. Available Online: https://apps.who.int/iris/handle/10665/330745 (Accessed on 27 August 2020).
Gonda, T. A., Kim, Y. I., Salas, M. C., Gamble, M. V., Shibata, W., Muthupalani, S., et al. (2012). Folic acid increases global DNA methylation and reduces inflammation to prevent helicobacter-associated gastric cancer in mice. Gastroenterology, 142(4), 824–33.e7. https://doi.org/10.1053/j.gastro.2011.12.058. Epub 20120113.
Sie, K. K., Medline, A., van Weel, J., Sohn, K. J., Choi, S. W., Croxford, R., et al. (2011). Effect of maternal and postweaning folic acid supplementation on colorectal cancer risk in the offspring. Gut, 60(12), 1687–1694. https://doi.org/10.1136/gut.2011.238782. Epub 20110511.
Ly, A., Lee, H., Chen, J., Sie, K. K., Renlund, R., Medline, A., et al. (2011). Effect of maternal and postweaning folic acid supplementation on mammary tumor risk in the offspring. Cancer Research, 71(3), 988–997. https://doi.org/10.1158/0008-5472.Can-10-2379. Epub 20101206.
Wei, E. K., Giovannucci, E., Selhub, J., Fuchs, C. S., Hankinson, S. E., & Ma, J. (2005). Plasma vitamin B6 and the risk of colorectal cancer and adenoma in women. Journal of the National Cancer Institute, 97(9), 684–692. https://doi.org/10.1093/jnci/dji116
Johanning, G. L., Heimburger, D. C., & Piyathilake, C. J. (2002). DNA methylation and diet in cancer. Journal of Nutrition, 132(12), 3814s-s3818. https://doi.org/10.1093/jn/132.12.3814S
Tryndyak, V. P., Han, T., Muskhelishvili, L., Fuscoe, J. C., Ross, S. A., Beland, F. A., & Pogribny, I. P. (2011). Coupling global methylation and gene expression profiles reveal key pathophysiological events in liver injury induced by a methyl-deficient diet. Molecular Nutrition and Food Research, 55(3), 411–418. https://doi.org/10.1002/mnfr.201000300. Epub 20101011.
Jiang, A., Wang, X., Shan, X., Li, Y., Wang, P., Jiang, P., & Feng, Q. (2015). Curcumin reactivates silenced tumor suppressor gene Rarβ by reducing DNA methylation. Phytotherapy Research, 29(8), 1237–1245. https://doi.org/10.1002/ptr.5373. Epub 20150515.
Lewinska, A., Adamczyk-Grochala, J., Deregowska, A., & Wnuk, M. (2017). Sulforaphane-induced cell cycle arrest and senescence are accompanied by DNA hypomethylation and changes in microrna profile in breast cancer cells. Theranostics, 7(14), 3461–77. https://doi.org/10.7150/thno.20657. Epub 20170815.
Ghazi, T., Arumugam, T., Foolchand, A., & Chuturgoon, A. A. (2020). The impact of natural dietary compounds and food-borne mycotoxins on DNA methylation and cancer. Cells, 9(9), 2004. https://doi.org/10.3390/cells9092004. Epub 20200831.
O’Brien, K. M., Sandler, D. P., Xu, Z., Kinyamu, H. K., Taylor, J. A., & Weinberg, C. R. (2018). Vitamin D, DNA methylation, and breast cancer. Breast Cancer Research, 20(1), 70. https://doi.org/10.1186/s13058-018-0994-y. Epub 20180711.
Pan, L., Matloob, A. F., Du, J., Pan, H., Dong, Z., Zhao, J., Feng, Y., Zhong, Y., Huang, B., & Lu, J. (2010). Vitamin D stimulates apoptosis in gastric cancer cells in synergy with trichostatin A/sodium butyrate-induced and 5-aza-2′-deoxycytidine-induced PTEN upregulation. The FEBS Journal, 277(4), 989–999. https://doi.org/10.1111/j.1742-4658.2009.07542.x. Epub 20100118.
Ng, K., Nimeiri, H. S., McCleary, N. J., Abrams, T. A., Yurgelun, M. B., Cleary, J. M., et al. (2019). Effect of high-dose Vs standard-dose vitamin D3 supplementation on progression-free survival among patients with advanced or metastatic colorectal cancer: The sunshine randomized clinical trial. JAMA, 321(14), 1370–1379. https://doi.org/10.1001/jama.2019.2402
Tapp, H. S., Commane, D. M., Bradburn, D. M., Arasaradnam, R., Mathers, J. C., Johnson, I. T., et al. (2013). Nutritional factors and gender influence age-related DNA methylation in the human rectal mucosa. Aging Cell, 12(1), 148–55. https://doi.org/10.1111/acel.12030. Epub 20121206.
Sofi, F., Cesari, F., Abbate, R., Gensini, G. F., & Casini, A. (2008). Adherence to mediterranean diet and health status: Meta-analysis. BMJ, 337, a1344. https://doi.org/10.1136/bmj.a1344. Epub 20080911.
Lorenzo, P. M., Izquierdo, A. G., Diaz-Lagares, A., Carreira, M. C., Macias-Gonzalez, M., Sandoval, J., Cueva, J., Lopez-Lopez, R., Casanueva, F. F., & Crujeiras, A. B. (2020). Znf577 methylation levels in leukocytes from women with breast cancer is modulated by adiposity, menopausal state, and the mediterranean diet. Frontiers in Endocrinology, 11, 245. https://doi.org/10.3389/fendo.2020.00245. Epub 20200423.
Mehta, R. S., Song, M., Nishihara, R., Drew, D. A., Wu, K., Qian, Z. R., et al. (2017). Dietary patterns and risk of colorectal cancer: Analysis by tumor location and molecular subtypes. Gastroenterology, 152(8), 1944–53.e1. https://doi.org/10.1053/j.gastro.2017.02.015. Epub 20170227.
Pussila, M., Sarantaus, L., Dermadi Bebek, D., Valo, S., Reyhani, N., Ollila, S., et al. (2013). Cancer-predicting gene expression changes in colonic mucosa of western diet Fed Mlh1+/- Mice. PLoS ONE, 8(10), e76865. https://doi.org/10.1371/journal.pone.0076865. Epub 20131008.
Zhang, F. F., Morabia, A., Carroll, J., Gonzalez, K., Fulda, K., Kaur, M., Vishwanatha, J. K., Santella, R. M., & Cardarelli, R. (2011). Dietary patterns are associated with levels of global genomic DNA methylation in a cancer-free population. The Journal of nutrition, 141(6), 1165–1171. https://doi.org/10.3945/jn.110.134536. Epub 20110427.
de Assis, S., Warri, A., Cruz, M. I., Laja, O., Tian, Y., Zhang, B., et al. (2012). High-Fat or Ethinyl-Oestradiol Intake During Pregnancy Increases Mammary Cancer Risk in Several Generations of Offspring. Nature Communications, 3, 1053. https://doi.org/10.1038/ncomms2058
Griswold, M. G., Fullman, N., Hawley, C., Arian, N., Zimsen, S. R., Tymeson, H. D., Venkateswaran, V., Tapp, A. D., Forouzanfar, M. H., Salama, J. S., & Abate, K. H. (2018). Alcohol use and burden for 195 countries and territories, 1990–2016: A systematic analysis for the global burden of disease study 2016. The Lancet, 392(10152), 1015–1035. https://doi.org/10.1016/s0140-6736(18)31310-2. Epub 20180823.
Rumgay, H., Shield, K., Charvat, H., Ferrari, P., Sornpaisarn, B., Obot, I., et al. (2021). Global burden of cancer in 2020 attributable to alcohol consumption: A population-based study. The Lancet Oncology, 22(8), 1071–1080. https://doi.org/10.1016/s1470-2045(21)00279-5
Bagnardi, V., Rota, M., Botteri, E., Tramacere, I., Islami, F., Fedirko, V., Scotti, L., Jenab, M., Turati, F., Pasquali, E., & Pelucchi, C. (2015). Alcohol consumption and site-specific cancer risk: A comprehensive dose–response meta-analysis. British Journal of Cancer, 112(3), 580–593. https://doi.org/10.1038/bjc.2014.579. Epub 20141125.
Seitz, H. K., & Stickel, F. (2007). Molecular mechanisms of alcohol-mediated carcinogenesis. Nature Reviews Cancer, 7(8), 599–612. https://doi.org/10.1038/nrc2191
Mato, J. M., Martínez-Chantar, M. L., & Lu, S. C. (2008). Methionine metabolism and liver disease. Annual Review of Nutrition, 28, 273–293. https://doi.org/10.1146/annurev.nutr.28.061807.155438
Kim, Y. I. (2005). Nutritional epigenetics: Impact of folate deficiency on DNA methylation and colon cancer susceptibility. Journal of Nutrition, 135(11), 2703–2709. https://doi.org/10.1093/jn/135.11.2703
Lambert, M. P., Paliwal, A., Vaissière, T., Chemin, I., Zoulim, F., Tommasino, M., Hainaut, P., Sylla, B., Scoazec, J. Y., Tost, J., & Herceg, Z. (2011). Aberrant DNA methylation distinguishes hepatocellular carcinoma associated with Hbv and Hcv infection and alcohol intake. Journal of Hepatology, 54(4), 705–715. https://doi.org/10.1016/j.jhep.2010.07.027. Epub 20100925.
Schernhammer, E. S., Giovannucci, E., Kawasaki, T., Rosner, B., Fuchs, C. S., & Ogino, S. (2010). Dietary folate, alcohol and B vitamins in relation to line-1 hypomethylation in colon cancer. Gut, 59(6), 794–9. https://doi.org/10.1136/gut.2009.183707. Epub 20091014.
Nishihara, R., Wang, M., Qian, Z. R., Baba, Y., Yamauchi, M., Mima, K., Sukawa, Y., Kim, S. A., Inamura, K., Zhang, X., & Wu, K. (2014). Alcohol, one-carbon nutrient intake, and risk of colorectal cancer according to tumor methylation level of Igf2 differentially methylated region. The American Journal of Clinical Nutrition, 100(6), 1479–1488. https://doi.org/10.3945/ajcn.114.095539. Epub 20141008.
van Engeland, M., Weijenberg, M. P., Roemen, G. M., Brink, M., de Bruïne, A. P., Goldbohm, R. A., et al. (2003). Effects of dietary folate and alcohol intake on promoter methylation in sporadic colorectal cancer: The Netherlands cohort study on diet and cancer. Cancer Research, 63(12), 3133–3137.
Giovannucci, E. (2004). Alcohol, one-carbon metabolism, and colorectal cancer: Recent insights from molecular studies. Journal of Nutrition, 134(9), 2475s-s2481. https://doi.org/10.1093/jn/134.9.2475S
Christensen, B. C., Kelsey, K. T., Zheng, S., Houseman, E. A., Marsit, C. J., Wrensch, M. R., et al. (2010). Breast cancer DNA methylation profiles are associated with tumor size and alcohol and folate intake. PLoS Genetics, 6(7), e1001043. https://doi.org/10.1371/journal.pgen.1001043. Epub 20100729.
Smith, I. M., Mydlarz, W. K., Mithani, S. K., & Califano, J. A. (2007). DNA global hypomethylation in squamous cell head and neck cancer associated with smoking, alcohol consumption and stage. International Journal of Cancer, 121(8), 1724–1728. https://doi.org/10.1002/ijc.22889
Chang, H. W., Ling, G. S., Wei, W. I., & Yuen, A. P. (2004). Smoking and drinking can induce P15 methylation in the upper aerodigestive tract of healthy individuals and patients with head and neck squamous cell carcinoma. Cancer, 101(1), 125–132. https://doi.org/10.1002/cncr.20323
Mani, S., Szymańska, K., Cuenin, C., Zaridze, D., Balassiano, K., Lima, S. C., et al. (2012). DNA methylation changes associated with risk factors in tumors of the upper aerodigestive tract. Epigenetics, 7(3), 270–277. https://doi.org/10.4161/epi.7.3.19306
Rider, C. F., & Carlsten, C. (2019). Air pollution and DNA methylation: Effects of exposure in humans. Clinical Epigenetics, 11(1), 1–15. https://doi.org/10.1186/s13148-019-0713-2. Epub 20190903.
Puett, R. C., Hart, J. E., Yanosky, J. D., Spiegelman, D., Wang, M., Fisher, J. A., et al. (2014). Particulate matter air pollution exposure, distance to road, and incident lung cancer in the nurses’ health study cohort. Environmental Health Perspectives, 122(9), 926–32. https://doi.org/10.1289/ehp.1307490. Epub 20140603.
Maghbooli, Z., Hossein-Nezhad, A., Adabi, E., Asadollah-Pour, E., Sadeghi, M., Mohammad-Nabi, S., et al. (2018). Air pollution during pregnancy and placental adaptation in the levels of global DNA methylation. PLoS ONE, 13(7), e0199772. https://doi.org/10.1371/journal.pone.0199772. Epub 20180706.
Somineni, H. K., Zhang, X., Myers, J. M. B., Kovacic, M. B., Ulm, A., Jurcak, N., Ryan, P. H., Hershey, G. K. K., & Ji, H. (2016). Ten-eleven translocation 1 (Tet1) methylation is associated with childhood asthma and traffic-related air pollution. Journal of Allergy and Clinical Immunology, 137(3), 797–805. https://doi.org/10.1016/j.jaci.2015.10.021. Epub 20151210.
Lamadema, N., Burr, S., & Brewer, A. C. (2019). Dynamic regulation of epigenetic demethylation by oxygen availability and cellular redox. Free Radical Biology and Medicine, 131, 282–98. https://doi.org/10.1016/j.freeradbiomed.2018.12.009. Epub 20181217.
Zhong, J., Karlsson, O., Wang, G., Li, J., Guo, Y., Lin, X., Zemplenyi, M., Sanchez-Guerra, M., Trevisi, L., Urch, B., & Speck, M. (2017). B vitamins attenuate the epigenetic effects of ambient fine particles in a pilot human intervention trial. Proceedings of the National Academy of Sciences, 114(13), 3503–3508. https://doi.org/10.1073/pnas.1618545114. Epub 20170313.
Tang, W. Y., Levin, L., Talaska, G., Cheung, Y. Y., Herbstman, J., Tang, D., et al. (2012). Maternal exposure to polycyclic aromatic hydrocarbons and 5’-Cpg methylation of interferon-Γ in cord white blood cells. Environment Health Perspectives, 120(8), 1195–200. https://doi.org/10.1289/ehp.1103744. Epub 20120504.
White, A. J., Chen, J., Teitelbaum, S. L., McCullough, L. E., Xu, X., Cho, Y. H., Conway, K., Beyea, J., Stellman, S. D., Steck, S. E., & Mordukhovich, I. (2016). Sources of polycyclic aromatic hydrocarbons are associated with gene-specific promoter methylation in women with breast cancer. Environmental Research, 145, 93–100. https://doi.org/10.1016/j.envres.2015.11.033. Epub 20151206.
Pavanello, S., Bollati, V., Pesatori, A. C., Kapka, L., Bolognesi, C., Bertazzi, P. A., et al. (2009). Global and gene-specific promoter methylation changes are related to Anti-B[a]Pde-DNA adduct levels and influence micronuclei levels in polycyclic aromatic hydrocarbon-exposed individuals. International Journal of Cancer, 125(7), 1692–1697. https://doi.org/10.1002/ijc.24492
Duan, H., He, Z., Ma, J., Zhang, B., Sheng, Z., Bin, P., Cheng, J., Niu, Y., Dong, H., Lin, H., & Dai, Y. (2013). Global and Mgmt promoter hypomethylation independently associated with genomic instability of lymphocytes in subjects exposed to high-dose polycyclic aromatic hydrocarbon. Archives of Toxicology, 87, 2013–2022. https://doi.org/10.1007/s00204-013-1046-0. Epub 20130330.
Palmisano, W. A., Divine, K. K., Saccomanno, G., Gilliland, F. D., Baylin, S. B., Herman, J. G., et al. (2000). Predicting lung cancer by detecting aberrant promoter methylation in sputum. Cancer Research, 60(21), 5954–5958.
Cipollini, M., Pastor, S., Gemignani, F., Castell, J., Garritano, S., Bonotti, A., Biarnés, J., Figlioli, G., Romei, C., Marcos, R., & Cristaudo, A. (2013). Tpo genetic variants and risk of differentiated thyroid carcinoma in two European populations. International Journal of Cancer, 133(12), 2843–2851. https://doi.org/10.1002/ijc.28317. Epub 20130713.
Huang, T., Chen, X., Hong, Q., Deng, Z., Ma, H., Xin, Y., Fang, Y., Ye, H., Wang, R., Zhang, C., & Ye, M. (2015). Meta-analyses of gene methylation and smoking behavior in non-small cell lung cancer patients. Scientific Reports, 5(1), 8897. https://doi.org/10.1038/srep08897. Epub 20150310.
Kettunen, E., Hernandez-Vargas, H., Cros, M. P., Durand, G., Le Calvez-Kelm, F., Stuopelyte, K., Jarmalaite, S., Salmenkivi, K., Anttila, S., Wolff, H., & Herceg, Z. (2017). Asbestos-associated genome-wide DNA methylation changes in lung cancer. International Journal of Cancer, 141(10), 2014–2029. https://doi.org/10.1002/ijc.30897. Epub 20170802.
Wang, Z., & Yang, C. (2019). Metal carcinogen exposure induces cancer stem cell-like property through epigenetic reprograming: a novel mechanism of metal carcinogenesis. Seminars in Cancer Biology, 57, 95–104. https://doi.org/10.1016/j.semcancer.2019.01.002. Epub 20190111.
Argos, M., Chen, L., Jasmine, F., Tong, L., Pierce, B. L., Roy, S., Paul-Brutus, R., Gamble, M. V., Harper, K. N., Parvez, F., & Rahman, M. (2015). Gene-specific differential DNA methylation and chronic arsenic exposure in an epigenome-wide association study of adults in Bangladesh. Environmental Health Perspectives, 123(1), 64–71. https://doi.org/10.1289/ehp.1307884. Epub 20141017.
Koestler, D. C., Avissar-Whiting, M., Houseman, E. A., Karagas, M. R., & Marsit, C. J. (2013). Differential DNA methylation in umbilical cord blood of infants exposed to low levels of arsenic in utero. Environmental Health Perspectives, 121(8), 971–977. https://doi.org/10.1289/ehp.1205925. Epub 20130611.
Braun, J. M. (2017). Early-life exposure to Edcs: Role in childhood obesity and neurodevelopment. Nature Reviews Endocrinology, 13(3), 161–173. https://doi.org/10.1038/nrendo.2016.186. Epub 20161118.
van den Dungen, M. W., Murk, A. J., Kampman, E., Steegenga, W. T., & Kok, D. E. (2017). Association between DNA methylation profiles in leukocytes and serum levels of persistent organic pollutants in dutch men. Environmental Epigenetics, 3(1), dvx001. https://doi.org/10.1093/eep/dvx001. Epub 20170227.
Benbrahim-Tallaa, L., Waterland, R. A., Dill, A. L., Webber, M. M., & Waalkes, M. P. (2007). Tumor suppressor gene inactivation during cadmium-induced malignant transformation of human prostate cells correlates with overexpression of De Novo DNA methyltransferase. Environmental Health Perspectives, 115(10), 1454–1459. https://doi.org/10.1289/ehp.10207
Zhou, Z. H., Lei, Y. X., & Wang, C. X. (2012). Analysis of aberrant methylation in DNA repair genes during malignant transformation of human bronchial epithelial cells induced by cadmium. Toxicology Science, 125(2), 412–7. https://doi.org/10.1093/toxsci/kfr320. Epub 20111123.
Ali, A. H., Kondo, K., Namura, T., Senba, Y., Takizawa, H., Nakagawa, Y., Toba, H., Kenzaki, K., Sakiyama, S., & Tangoku, A. (2011). Aberrant DNA methylation of some tumor suppressor genes in lung cancers from workers with chromate exposure. Molecular Carcinogenesis, 50(2), 89–99. https://doi.org/10.1002/mc.20697. Epub 20101123.
Zheng, H., Xing, X., Hu, T., Zhang, Y., Zhang, J., Zhu, G., Li, Y., & Qi, S. (2018). Biomass burning contributed most to the human cancer risk exposed to the soil-bound Pahs from Chengdu economic region, Western China. Ecotoxicology and Environmental Safety, 159, 63–70. https://doi.org/10.1016/j.ecoenv.2018.04.065. Epub 20180503.
Kim, K. Y., Kim, D. S., Lee, S. K., Lee, I. K., Kang, J. H., Chang, Y. S., et al. (2010). Association of low-dose exposure to persistent organic pollutants with global DNA hypomethylation in healthy Koreans. Environment Health Perspective, 118(3), 370–4. https://doi.org/10.1289/ehp.0901131. Epub 20091106.
Rusiecki, J. A., Baccarelli, A., Bollati, V., Tarantini, L., Moore, L. E., & Bonefeld-Jorgensen, E. C. (2008). Global DNA hypomethylation is associated with high serum-persistent organic pollutants in greenlandic inuit. Environment Health Perspective, 116(11), 1547–52. https://doi.org/10.1289/ehp.11338. Epub 20080716.
Kile, M. L., Baccarelli, A., Hoffman, E., Tarantini, L., Quamruzzaman, Q., Rahman, M., et al. (2012). Prenatal arsenic exposure and DNA methylation in maternal and umbilical cord blood leukocytes. Environment Health Perspective, 120(7), 1061–6. https://doi.org/10.1289/ehp.1104173. Epub 20120330.
Intarasunanont, P., Navasumrit, P., Waraprasit, S., Chaisatra, K., Suk, W. A., Mahidol, C., et al. (2012). Effects of arsenic exposure on DNA methylation in cord blood samples from newborn babies and in a human lymphoblast cell line. Environment Health, 11, 31. https://doi.org/10.1186/1476-069x-11-31. Epub 20120502.
Wang, Y., Scheiber, M. N., Neumann, C., Calin, G. A., & Zhou, D. (2011). Microrna regulation of ionizing radiation-induced premature senescence. International Journal of Radiation Oncology Biology Physics, 81(3), 839–48. https://doi.org/10.1016/j.ijrobp.2010.09.048. Epub 20101117.
Koturbash, I., Rugo, R. E., Hendricks, C. A., Loree, J., Thibault, B., Kutanzi, K., et al. (2006). Irradiation induces DNA damage and modulates epigenetic effectors in distant bystander tissue in vivo. Oncogene, 25(31), 4267–75. https://doi.org/10.1038/sj.onc.1209467. Epub 20060313.
Koturbash, I., Pogribny, I., & Kovalchuk, O. (2005). Stable loss of global DNA methylation in the radiation-target tissue—A possible mechanism contributing to radiation carcinogenesis? Biochemical and Biophysical Research Communications, 337(2), 526–533. https://doi.org/10.1016/j.bbrc.2005.09.084. Epub 20050922.
Loree, J., Koturbash, I., Kutanzi, K., Baker, M., Pogribny, I., & Kovalchuk, O. (2006). Radiation-induced molecular changes in rat mammary tissue: Possible implications for radiation-induced carcinogenesis. International Journal of Radiation Biology, 82(11), 805–815. https://doi.org/10.1080/09553000600960027
Vandiver, A. R., Irizarry, R. A., Hansen, K. D., Garza, L. A., Runarsson, A., Li, X., et al. (2015). Age and sun exposure-related widespread genomic blocks of hypomethylation in nonmalignant skin. Genome Biology, 16(1), 80. https://doi.org/10.1186/s13059-015-0644-y. Epub 20150416.
Arbiser, J. L., Fan, C. Y., Su, X., Van Emburgh, B. O., Cerimele, F., Miller, M. S., et al. (2004). Involvement of P53 and P16 tumor suppressor genes in recessive dystrophic epidermolysis bullosa-associated squamous cell carcinoma. The Journal of Investigative Dermatology, 123(4), 788–790. https://doi.org/10.1111/j.0022-202X.2004.23418.x
Lento, W., Congdon, K., Voermans, C., Kritzik, M., & Reya, T. (2013). Wnt signaling in normal and malignant hematopoiesis. Cold Spring Harbor Perspectives in Biology, 5(2), a008011. https://doi.org/10.1101/cshperspect.a008011. Epub 20130201.
Yamamoto, M. L., Maier, I., Dang, A. T., Berry, D., Liu, J., Ruegger, P. M., et al. (2013). Intestinal bacteria modify lymphoma incidence and latency by affecting systemic inflammatory state, oxidative stress, and leukocyte genotoxicity. Cancer Research, 73(14), 4222–4232. https://doi.org/10.1158/0008-5472.Can-13-0022
Round, J. L., Lee, S. M., Li, J., Tran, G., Jabri, B., Chatila, T. A., et al. (2011). The toll-like receptor 2 pathway establishes colonization by a commensal of the human microbiota. Science, 332(6032), 974–7. https://doi.org/10.1126/science.1206095. Epub 20110421.
Schaible, T. D., Harris, R. A., Dowd, S. E., Smith, C. W., & Kellermayer, R. (2011). Maternal methyl-donor supplementation induces prolonged murine offspring colitis susceptibility in association with mucosal epigenetic and microbiomic changes. Human Molecular Genetics, 20(9), 1687–1696. https://doi.org/10.1093/hmg/ddr044. Epub 20110204.
Rossi, M., Amaretti, A., & Raimondi, S. (2011). Folate production by probiotic bacteria. Nutrients, 3(1), 118–34. https://doi.org/10.3390/nu3010118. Epub 20110118.
Lin, C. C. J., & Wang, M. C. (2017). Microbial metabolites regulate host lipid metabolism through NR5A–Hedgehog signalling. Nature Cell Biology, 19(5), 550–557. https://doi.org/10.1038/ncb3515. Epub 20170424.
Romano, K. A., Martinez-del Campo, A., Kasahara, K., Chittim, C. L., Vivas, E. I., Amador-Noguez, D., Balskus, E. P., & Rey, F. E. (2017). Metabolic, epigenetic, and transgenerational effects of gut bacterial choline consumption. Cell Host and Microbe, 22(3), 279–290. https://doi.org/10.1016/j.chom.2017.07.021. Epub 20170824.
Takahashi, K., Sugi, Y., Nakano, K., Tsuda, M., Kurihara, K., Hosono, A., & Kaminogawa, S. (2011). Epigenetic control of the host gene by commensal bacteria in large intestinal epithelial cells. Journal of Biological Chemistry, 286(41), 35755–35762. https://doi.org/10.1074/jbc.M111.271007. Epub 20110823.
Remely, M., Ferk, F., Sterneder, S., Setayesh, T., Roth, S., Kepcija, T., et al. (2017). Egcg prevents high fat diet-induced changes in gut microbiota, decreases of DNA strand breaks, and changes in expression and DNA methylation of Dnmt1 and Mlh1 in C57bl/6j Male Mice. Oxidative Medicine and Cellular Longevity, 2017, 3079148. https://doi.org/10.1155/2017/3079148. Epub 20170104.
Wu, Y., Wang, C. Z., Wan, J. Y., Yao, H., & Yuan, C. S. (2021). Dissecting the interplay mechanism between epigenetics and gut microbiota: Health maintenance and disease prevention. International Journal of Molecular Sciences, 22(13), 6933. https://doi.org/10.3390/ijms22136933. Epub 20210628.
Soda, K., Kano, Y., Chiba, F., Koizumi, K., & Miyaki, Y. (2013). Increased polyamine intake inhibits age-associated alteration in global DNA methylation and 1,2-dimethylhydrazine-induced tumorigenesis. PLoS ONE, 8(5), e64357. https://doi.org/10.1371/journal.pone.0064357. Epub 20130516.
Bind, M. A., Zanobetti, A., Gasparrini, A., Peters, A., Coull, B., Baccarelli, A., et al. (2014). Effects of temperature and relative humidity on DNA methylation. Epidemiology, 25(4), 561–569. https://doi.org/10.1097/ede.0000000000000120
Xu, R., Li, S., Li, S., Wong, E. M., Southey, M. C., Hopper, J. L., Abramson, M. J., & Guo, Y. (2021). Ambient temperature and genome-wide DNA methylation: A twin and family study in Australia. Environmental Pollution, 285, 117700. https://doi.org/10.1016/j.envpol.2021.117700. Epub 20210701.
Wang, Z., Wilson, C. M., Mendelev, N., Ge, Y., Galfalvy, H., Elder, G., Ahlers, S., Yarnell, A. M., LoPresti, M. L., Kamimori, G. H., & Carr, W. (2020). Acute and chronic molecular signatures and associated symptoms of blast exposure in military breachers. Journal of Neurotrauma, 37(10), 1221–1232. https://doi.org/10.1089/neu.2019.6742. Epub 20191212.
Barboza, E. P., Cirach, M., Khomenko, S., Iungman, T., Mueller, N., Barrera-Gómez, J., et al. (2021). Green space and mortality in European cities: A health impact assessment study. Lancet Planet Health, 5(10), e718–e730. https://doi.org/10.1016/s2542-5196(21)00229-1
Xu, R., Li, S., Li, S., Wong, E. M., Southey, M. C., Hopper, J. L., Abramson, M. J., & Guo, Y. (2021). Residential surrounding greenness and DNA methylation: An epigenome-wide association study. Environment International, 154, 106556. https://doi.org/10.1016/j.envint.2021.106556. Epub 20210413.
Haim, A., & Zubidat, A. E. (2015). Artificial light at night: Melatonin as a mediator between the environment and epigenome. Philosophical Transactions of the Royal Society B: Biological Sciences, 370(1667), 20140121. https://doi.org/10.1098/rstb.2014.0121
Anderson, O. S., Sant, K. E., & Dolinoy, D. C. (2012). Nutrition and epigenetics: An interplay of dietary methyl donors, one-carbon metabolism and DNA methylation. The Journal of Nutritional Biochemistry, 23(8), 853–859. https://doi.org/10.1016/j.jnutbio.2012.03.003. Epub 20120627.
Heijmans, B. T., Tobi, E. W., Stein, A. D., Putter, H., Blauw, G. J., Susser, E. S., Slagboom, P. E., & Lumey, L. H. (2008). Persistent epigenetic differences associated with prenatal exposure to famine in humans. Proceedings of the National Academy of Sciences, 105(44), 17046–17049. https://doi.org/10.1073/pnas.0806560105
Cai, J., Zhao, Y., Liu, P., Xia, B., Zhu, Q., Wang, X., Song, Q., Kan, H., & Zhang, Y. (2017). Exposure to Particulate Air Pollution During Early Pregnancy Is Associated with Placental DNA Methylation. Science of The Total Environment, 607, 1103–1108. https://doi.org/10.1016/j.scitotenv.2017.07.029
Coker, E. S., Gunier, R., Huen, K., Holland, N., & Eskenazi, B. (2018). DNA methylation and socioeconomic status in a Mexican-American birth cohort. Clinical Epigenetics, 10, 1–12. https://doi.org/10.1186/s13148-018-0494-z. Epub 20180508.
Smith, J. A., Zhao, W., Wang, X., Ratliff, S. M., Mukherjee, B., Kardia, S. L. R., et al. (2017). Neighborhood characteristics influence DNA methylation of genes involved in stress response and inflammation: The multi-ethnic study of atherosclerosis. Epigenetics, 12(8), 662–73. https://doi.org/10.1080/15592294.2017.1341026. Epub 20170705.
Agrawal, K., Das, V., Vyas, P., & Hajdúch, M. (2018). Nucleosidic DNA demethylating epigenetic drugs–a comprehensive review from discovery to clinic. Pharmacology and Therapeutics, 188, 45–79. https://doi.org/10.1016/j.pharmthera.2018.02.006. Epub 20180215.
Flotho, C., Sommer, S., & Lübbert, M. (2018). DNA-hypomethylating agents as epigenetic therapy before and after allogeneic hematopoietic stem cell transplantation in myelodysplastic syndromes and juvenile myelomonocytic leukemia. Seminars in Cancer Biology, 51, 68–79. https://doi.org/10.1016/j.semcancer.2017.10.011. Epub 20171109.
Greenberg, M. V., & Bourc’his, D. (2019). The diverse roles of DNA methylation in mammalian development and disease. Nature Reviews Molecular Cell Biology, 20(10), 590–607. https://doi.org/10.1038/s41580-019-0159-6. Epub 20190809.
Vernier, M., McGuirk, S., Dufour, C. R., Wan, L., Audet-Walsh, E., St-Pierre, J., et al. (2020). Inhibition of Dnmt1 and Errα crosstalk suppresses breast cancer via derepression of Irf4. Oncogene, 39(41), 6406–20. https://doi.org/10.1038/s41388-020-01438-1. Epub 20200827.
Harlid, S., Xu, Z., Kirk, E., Wilson, L. E., Troester, M. A., & Taylor, J. A. (2019). Hormone therapy use and breast tissue DNA methylation: Analysis of epigenome wide data from the normal breast study. Epigenetics, 14(2), 146–57. https://doi.org/10.1080/15592294.2019.1580111. Epub 20190301.
Xiong, G., Yao, L., Hong, P., Yang, L., Ci, W., Liu, L., et al. (2018). Aristolochic acid containing herbs induce gender-related oncological differences in upper tract urothelial carcinoma patients. Cancer Management and Research, 10, 6627–39. https://doi.org/10.2147/cmar.S178554. Epub 20181204.
Huang, Y. T., Wu, T. S., Lu, C. C., Yu, F. Y., & Liu, B. H. (2018). Aristolochic acid I interferes with the expression of blcap tumor suppressor gene in human cells. Toxicology Letters, 291, 129–37. https://doi.org/10.1016/j.toxlet.2018.03.032. Epub 20180412.
Poon, S. L., Huang, M. N., Choo, Y., McPherson, J. R., Yu, W., Heng, H. L., et al. (2015). Mutation signatures implicate aristolochic acid in bladder cancer development. Genome Medicine, 7(1), 38. https://doi.org/10.1186/s13073-015-0161-3. Epub 20150428.
Fang, M. Z., Wang, Y., Ai, N., Hou, Z., Sun, Y., Lu, H., et al. (2003). Tea polyphenol (-)-Epigallocatechin-3-gallate inhibits DNA methyltransferase and reactivates methylation-silenced genes in cancer cell lines. Cancer Research, 63(22), 7563–7570.
Nandakumar, V., Vaid, M., & Katiyar, S. K. (2011). (-)-Epigallocatechin-3-gallate reactivates silenced tumor suppressor genes, Cip1/P21 and P16ink4a, by reducing DNA methylation and increasing histones acetylation in human skin cancer cells. Carcinogenesis, 32(4), 537–44. https://doi.org/10.1093/carcin/bgq285. Epub 20110105.
Pandey, M., Shukla, S., & Gupta, S. (2010). Promoter demethylation and chromatin remodeling by green tea polyphenols leads to re-expression of Gstp1 in human prostate cancer cells. International Journal of Cancer, 126(11), 2520–2533. https://doi.org/10.1002/ijc.24988
Sheng, J., Shi, W., Guo, H., Long, W., Wang, Y., Qi, J., Liu, J., & Xu, Y. (2019). The inhibitory effect of (−)-epigallocatechin-3-gallate on breast cancer progression via reducing SCUBE2 methylation and DNMT activity. Molecules, 24(16), 2899. https://doi.org/10.3390/molecules24162899. Epub 20190809.
Berletch, J. B., Liu, C., Love, W. K., Andrews, L. G., Katiyar, S. K., & Tollefsbol, T. O. (2008). Epigenetic and genetic mechanisms contribute to telomerase inhibition by Egcg. Journal of Cellular Biochemistry, 103(2), 509–519. https://doi.org/10.1002/jcb.21417
Lee, W. J., & Zhu, B. T. (2006). Inhibition of DNA methylation by caffeic acid and chlorogenic acid two common catechol-containing coffee polyphenols. Carcinogenesis, 27(2), 269–77. https://doi.org/10.1093/carcin/bgi206. Epub 20050804.
Karabegović, I., Portilla-Fernandez, E., Li, Y., Ma, J., Maas, S. C., Sun, D., Hu, E. A., Kühnel, B., Zhang, Y., Ambatipudi, S., & Fiorito, G. (2021). Epigenome-wide association meta-analysis of DNA methylation with coffee and tea consumption. Nature Communications, 12(1), 2830. https://doi.org/10.1038/s41467-021-22752-6. Epub 20210514.
Cedernaes, J., Osler, M. E., Voisin, S., Broman, J. E., Vogel, H., Dickson, S. L., et al. (2015). Acute sleep loss induces tissue-specific epigenetic and transcriptional alterations to circadian clock genes in men. Journal of Clinical Endocrinology and Metabolism, 100(9), E1255-61. https://doi.org/10.1210/jc.2015-2284. Epub 20150713.
Plante, D. T., Papale, L. A., Madrid, A., Cook, J. D., Prairie, M. L., & Alisch, R. S. (2021). Pax8/Pax8-As1 DNA methylation levels are associated with objective sleep duration in persons with unexplained hypersomnolence using a deep phenotyping approach. Sleep. https://doi.org/10.1093/sleep/zsab108
Jansen, E. C., Dolinoy, D. C., O’Brien, L. M., Peterson, K. E., Chervin, R. D., Banker, M., et al. (2019). Sleep duration and fragmentation in relation to leukocyte DNA methylation in adolescents. Sleep. https://doi.org/10.1093/sleep/zsz121
Hopkins, B. D., Goncalves, M. D., & Cantley, L. C. (2016). Obesity and cancer mechanisms: Cancer metabolism. Journal of Clinical Oncology, 34(35), 4277. https://doi.org/10.1200/jco.2016.67.9712. Epub 20161107.
Gu, Y., Zhang, C. W. H., Wang, L., Zhao, Y., Wang, H., Ye, Q., & Gao, S. (2018). Association analysis between body mass index and genomic DNA methylation across 15 major cancer types. Journal of Cancer, 9(14), 2532. https://doi.org/10.7150/jca.23535. Epub 20180622.
Mendoza-Pérez, J., Gu, J., Herrera, L. A., Tannir, N. M., Zhang, S., Matin, S., et al. (2017). Prognostic significance of promoter Cpg island methylation of obesity-related genes in patients with nonmetastatic renal cell carcinoma. Cancer, 123(18), 3617–27. https://doi.org/10.1002/cncr.30707. Epub 20170523.
Yan, F., Shen, N., Pang, J. X., Zhang, Y. W., Rao, E. Y., Bode, A. M., et al. (2017). Fatty acid-binding protein Fabp4 mechanistically links obesity with aggressive Aml by enhancing aberrant DNA methylation in Aml cells. Leukemia, 31(6), 1434–42. https://doi.org/10.1038/leu.2016.349. Epub 20161125.
Zhang, T. J., Xu, Z. J., Gu, Y., Ma, J. C., Wen, X. M., Zhang, W., et al. (2021). Identification and validation of obesity-related gene Lep methylation as a prognostic indicator in patients with acute myeloid leukemia. Clinical Epigenetics, 13(1), 16. https://doi.org/10.1186/s13148-021-01013-9
Huang, H., Hu, G., Cai, J., Xia, B., Liu, J., Li, X., Gao, W., Zhang, J., Liu, Y., & Zhuang, Z. (2014). Role of poly (ADP-ribose) glycohydrolase silencing in DNA hypomethylation induced by benzo (a) pyrene. Biochemical and Biophysical Research Communications, 452(3), 708–714. https://doi.org/10.1016/j.bbrc.2014.08.146. Epub 20140906.
Xing, C., Wang, Q. F., Li, B., Tian, H., Ni, Y., Yin, S., & Li, G. (2010). Methylation and expression analysis of tumor suppressor genes P15 and P16 in benzene poisoning. Chemico-Biological Interactions, 184(1–2), 306–309. https://doi.org/10.1016/j.cbi.2009.12.028. Epub 20100104.
Zhang, A., Li, H., Xiao, Y., Chen, L., Zhu, X., Li, J., et al. (2017). Aberrant methylation of nucleotide excision repair genes is associated with chronic arsenic poisoning. Biomarkers, 22(5), 429–38. https://doi.org/10.1080/1354750x.2016.1217933. Epub 20160812.
Nielsen, C. H., Larsen, A., & Nielsen, A. L. (2016). DNA methylation alterations in response to prenatal exposure of maternal cigarette smoking: A persistent epigenetic impact on health from maternal lifestyle? Archives of Toxicology, 90, 231–245. https://doi.org/10.1007/s00204-014-1426-0. Epub 20141206.
Soubry, A., Schildkraut, J. M., Murtha, A., Wang, F., Huang, Z., Bernal, A., et al. (2013). Paternal obesity is associated with Igf2 hypomethylation in newborns: Results from a newborn epigenetics study (Nest) cohort. BMC Medicine, 11, 29. https://doi.org/10.1186/1741-7015-11-29. Epub 20130206.
Tando, Y., Hiura, H., Takehara, A., Ito-Matsuoka, Y., Arima, T., & Matsui, Y. (2021). Epi-mutations for spermatogenic defects by maternal exposure to Di(2-Ethylhexyl) phthalate. eLife. https://doi.org/10.7554/eLife.70322. Epub 20210728.
Soubry, A., Hoyo, C., Butt, C. M., Fieuws, S., Price, T. M., Murphy, S. K., et al. (2017). Human exposure to flame-retardants is associated with aberrant DNA methylation at imprinted genes in sperm. Environmental Epigenetics, 3(1), dvx003. https://doi.org/10.1093/eep/dvx003. Epub 20170414.
Koturbash, I., Baker, M., Loree, J., Kutanzi, K., Hudson, D., Pogribny, I., et al. (2006). Epigenetic dysregulation underlies radiation-induced transgenerational genome instability in vivo. International Journal of Radiation Oncology Biology Physics, 66(2), 327–330. https://doi.org/10.1016/j.ijrobp.2006.06.012
McGowan, P. O., Sasaki, A., D’Alessio, A. C., Dymov, S., Labonté, B., Szyf, M., et al. (2009). Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nature Neuroscience, 12(3), 342–348. https://doi.org/10.1038/nn.2270
Yehuda, R., Daskalakis, N. P., Bierer, L. M., Bader, H. N., Klengel, T., Holsboer, F., & Binder, E. B. (2016). Holocaust exposure induced intergenerational effects on Fkbp5 methylation. Biological Psychiatry, 80(5), 372–380. https://doi.org/10.1016/j.biopsych.2015.08.005. Epub 20150812.
Rakoff-Nahoum, S., & Medzhitov, R. (2009). Toll-like receptors and cancer. Nature Reviews Cancer, 9(1), 57–63. https://doi.org/10.1038/nrc2541. Epub 20081204.
Paschos, K., & Allday, M. J. (2010). Epigenetic reprogramming of host genes in viral and microbial pathogenesis. Trends Microbiology, 18(10), 439–47. https://doi.org/10.1016/j.tim.2010.07.003. Epub 20100818.
Cheung, C. C., Chung, G. T., Lun, S. W., To, K. F., Choy, K. W., Lau, K. M., et al. (2014). Mir-31 Is consistently inactivated in Ebv-associated nasopharyngeal carcinoma and contributes to its tumorigenesis. Molecular Cancer, 13, 184. https://doi.org/10.1186/1476-4598-13-184. Epub 20140807.
Platt, G., Carbone, A., & Mittnacht, S. (2002). P16ink4a loss and sensitivity in Kshv associated primary effusion lymphoma. Oncogene, 21(12), 1823–1831. https://doi.org/10.1038/sj.onc.1205360
Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature, 517(7536), 576-582. https://doi.org/10.1038/nature14129.
Wilting, S. M., van Boerdonk, R. A., Henken, F. E., Meijer, C. J., Diosdado, B., Meijer, G. A., et al. (2010). Methylation-mediated silencing and tumour suppressive function of Hsa-Mir-124 in cervical cancer. Molecular Cancer, 9, 167. https://doi.org/10.1186/1476-4598-9-167
Xie, Q., Chen, L., Shan, X., Shan, X., Tang, J., Zhou, F., Chen, Q., Quan, H., Nie, D., Zhang, W., & Huang, A. L. (2014). Epigenetic silencing of Sfrp1 and Sfrp5 by hepatitis B virus X protein enhances hepatoma cell tumorigenicity through Wnt signaling pathway. International Journal of Cancer, 135(3), 635–646. https://doi.org/10.1002/ijc.28697. Epub 20140113.
Lee, S. M., Lee, Y. G., Bae, J. B., Choi, J. K., Tayama, C., Hata, K., Yun, Y., Seong, J. K., & Kim, Y. J. (2014). Hbx induces hypomethylation of distal intragenic Cpg islands required for active expression of developmental regulators. Proceedings of the National Academy of Sciences, 111(26), 9555–9560. https://doi.org/10.1073/pnas.1400604111. Epub 20140618.
Fan, H., Zhang, H., Pascuzzi, P. E., & Andrisani, O. (2016). Hepatitis B Virus X protein induces epcam expression via active DNA demethylation directed by rela in complex with Ezh2 and Tet2. Oncogene, 35(6), 715–26. https://doi.org/10.1038/onc.2015.122. Epub 20150420.
Kuss-Duerkop, S. K., Westrich, J. A., & Pyeon, D. (2018). DNA tumor virus regulation of host DNA methylation and its implications for immune evasion and oncogenesis. Viruses. https://doi.org/10.3390/v10020082. Epub 20180213.
Xiong, W. M., Xu, Q. P., Xiao, R. D., Hu, Z. J., Cai, L., & He, F. (2019). Genome-wide DNA methylation and Rna expression profiles identified Ripk3 as a differentially methylated gene in chlamydia pneumoniae infection lung carcinoma patients in China. Cancer Management and Research, 11, 5785–97. https://doi.org/10.2147/cmar.S186217. Epub 20190628.
Kessler, M., Hoffmann, K., Fritsche, K., Brinkmann, V., Mollenkopf, H. J., Thieck, O., Teixeira da Costa, A. R., Braicu, E. I., Sehouli, J., Mangler, M., & Berger, H. (2019). Chronic chlamydia infection in human organoids increases stemness and promotes age-dependent Cpg methylation. Nature Communications, 10(1), 1194. https://doi.org/10.1038/s41467-019-09144-7. Epub 20190318.
Choung, H. K., Kim, Y. A., Lee, M. J., Kim, N., & Khwarg, S. I. (2012). Multigene methylation analysis of ocular adnexal malt lymphoma and their relationship to chlamydophila psittaci infection and clinical characteristics in South Korea. Investigative Ophthalmology and Visual Science, 53(4), 1928–1935. https://doi.org/10.1167/iovs.11-7668. Epub 20120406.
Niwa, T., Tsukamoto, T., Toyoda, T., Mori, A., Tanaka, H., Maekita, T., et al. (2010). Inflammatory processes triggered by helicobacter pylori infection cause aberrant DNA methylation in gastric epithelial cells. Cancer Research, 70(4), 1430–40. https://doi.org/10.1158/0008-5472.Can-09-2755. Epub 20100202.
Huang, F. Y., Chan, A. O., Rashid, A., Wong, D. K., Cho, C. H., & Yuen, M. F. (2012). Helicobacter pylori induces promoter methylation of E-cadherin via interleukin-1β activation of nitric oxide production in gastric cancer cells. Cancer, 118(20), 4969–80. https://doi.org/10.1002/cncr.27519. Epub 20120313.
Perri, F., Cotugno, R., Piepoli, A., Merla, A., Quitadamo, M., Gentile, A., et al. (2007). Aberrant DNA methylation in non-neoplastic gastric mucosa of H. pylori infected patients and effect of eradication. Official Journal of the American College of Gastroenterology ACG., 102(7), 1361–71. https://doi.org/10.1111/j.1572-0241.2007.01284.x. Epub 20070517.
Sepulveda, A. R., Yao, Y., Yan, W., Park, D. I., Kim, J. J., Gooding, W., et al. (2010). Cpg methylation and reduced expression of O6-methylguanine DNA methyltransferase is associated with helicobacter pylori infection. Gastroenterology, 138(5), 1836–44. https://doi.org/10.1053/j.gastro.2009.12.042. Epub 20100104.
Peterson, A. J., Menheniott, T. R., O’Connor, L., Walduck, A. K., Fox, J. G., Kawakami, K., et al. (2010). Helicobacter pylori infection promotes methylation and silencing of trefoil factor 2 leading to gastric tumor development in mice and humans. Gastroenterology, 139(6), 2005–17. https://doi.org/10.1053/j.gastro.2010.08.043. Epub 20100827.
Ravikumar, B., Sarkar, S., Davies, J. E., Futter, M., Garcia-Arencibia, M., Green-Thompson, Z. W., et al. (2010). Regulation of mammalian autophagy in physiology and pathophysiology. Physiological Reviews, 90(4), 1383–1435. https://doi.org/10.1152/physrev.00030.2009
Maekita, T., Nakazawa, K., Mihara, M., Nakajima, T., Yanaoka, K., Iguchi, M., et al. (2006). High levels of aberrant DNA methylation in helicobacter pylori-infected gastric mucosae and its possible association with gastric cancer risk. Clinical Cancer Research, 12(3 Pt 1), 989–995. https://doi.org/10.1158/1078-0432.Ccr-05-2096
Gutiérrez, M. I., Siraj, A. K., Khaled, H., Koon, N., El-Rifai, W., & Bhatia, K. (2004). Cpg island methylation in schistosoma- and non-schistosoma-associated bladder cancer. Modern Pathology, 17(10), 1268–1274. https://doi.org/10.1038/modpathol.3800177
Amonoo, H. L., El-Jawahri, A., Deary, E. C., Traeger, L. N., Cutler, C. S., Antin, J. A., Huffman, J. C., & Lee, S. J. (2022). Yin and Yang of psychological health in the cancer experience: Does positive psychology have a role? Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology, 40(22), 2402–2407. https://doi.org/10.1200/jco.21.02507. Epub 20220404.
Ortiz-Barahona, V., Joshi, R. S., & Esteller, M. (2022). Use of DNA methylation profiling in translational oncology. Seminar Cancer Biology, 83, 523–35. https://doi.org/10.1016/j.semcancer.2020.12.011. Epub 20201219.
Funding
This work was supported by the Fundamental Research Funds for the Central Universities [lzujbky-2022-sp08]; Project of Gansu Provincial Department of Education [2021jyjbgs-02]; Project of Gansu Provincial Development and Reform Commission [2020–2022]; Major Science and Technology Project of Gansu Province [20zd7fa003]; Medical Innovation and Development Project of Lanzhou University [lzuyxcx-2022–154, lzuyxcx-2022–141].
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. J. L. performed data collection, literature collection, and manuscript writing. B. H. and F. D. participated in the investigation and revision. Y. L. designed, supervised, and guided this study. All authors contributed to the article and approved the submitted version.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, J., Huang, B., Ding, F. et al. Environment factors, DNA methylation, and cancer. Environ Geochem Health 45, 7543–7568 (2023). https://doi.org/10.1007/s10653-023-01749-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-023-01749-8