Abstract
Primary bone and joint cancers are rare and understudied, yet these neoplasms are difficult to treat and impact all age groups. To explore the long-term changes in the occurrence of bone and joint cancers, patients diagnosed with these neoplasms between 1975 and 2016 were identified in the Surveillance Epidemiology and End Results of the National Cancer Institute of the USA. The age-adjusted incidence (AAIR) and mortality (AAMR) rates were calculated for three decades and compared to AAIR and AAMR in years 1975–1984. By using the population-based cancer registries of the USA, Iowa was identified as a state with increased cases of bone and joint malignancies. The bone and joint cancer cases in Iowa were correlated with the percentage of rural population, the average farmland size, or the residential radon levels. Results demonstrated that the mean AAIR of bone and joint cancers for US female and male patients (< 50 years of age) increased from 0.57 (95% C.I. 0.55–0.63) and 0.76 (95% C.I. 0.69–0.82) for years 1975–1984 to 0.71 (95% C.I. 0.66–0.76) and 0.94 (95% C.I. 0.87–1.07) for years 2005–2014, respectively. The increase in bone and joint cancer cases in Iowa positively correlated with the percentage rural population (R = 0.222, P < 0.02), and the average farmland size (R = 0.236, P < 0.02) but not the radon levels (R = − 0.038, P < 0.7). The findings revealed that patients younger than 50 years of age and those who resided in rural areas and engaged in farming were more likely to be diagnosed with primary bone and joint cancers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rare cancers receive less scientific attention because they affect fewer than 15 patients per 100,000 per year. Collectively, they comprise between 60 and 71 different cancer sites and account for 25% of all reported malignancies in the USA (Greenlee et al., 2010). Primary bone and joint tumors account for less than 0.2% of all US cancer cases (Siegel et al., 2020), a seemingly small proportion; however, these cancers represent significant health burdens as they impact all age groups and are difficult to treat malignancies (Dai et al., 2011).
The most common histological subtypes of bone and joint malignancies are osteosarcoma, chondrosarcoma, and Ewing sarcoma (Damron et al., 2007). Osteosarcoma and Ewing sarcomas are primary bone tumors that contribute to around 6% of all childhood and adolescent malignancies in the USA (Heare et al., 2009; Ottaviani & Jaffe, 2009). In contrast, chondrosarcoma is a primary bone sarcoma that develops from cartilage cells and is more common among middle-aged individuals with 30–60 years of age being the average age for diagnosis (Gelderblom et al., 2008). It accounts for approximately 40% of the primary bone cancers in this population (Zamborsky et al., 2019). Chondrosarcoma is a difficult to treat cancer as it is largely chemo- and radiotherapy resistant (David et al., 2011). Osteosarcoma and Ewing sarcoma respond better to chemo- and radiotherapy; however, patients that suffer from metastatic and recurrent diseases have a survival rate < 30% and their treatment options have not changed substantially over the years (Grunewald et al., 2018; Otoukesh et al., 2018; Rathore & Van Tine, 2021).
Evidence from epidemiological studies suggest that exposure to environmental factors, such as radon, pesticides, diesel exhaust fumes, and other hazards positively correlates with the incidence of cancer (Burns & Juberg, 2021; Cohen et al., 2019; Weichenthal et al., 2012). Radon is the primary cause of lung cancer in non-smokers and is estimated to contribute to 3–20% of lung cancer deaths worldwide (Kim et al., 2016; Stanley et al., 2019). Lymphohematopoietic cancers, such as lymphomas, leukemias, and myelomas, are linked to exposure to pesticides and biological agents (Alexander et al., 2007; Koff et al., 2015; Patel et al., 2020). Other common cancers including breast, prostate, and colorectal cancers also demonstrate a positive relationship with exposure to pesticides (Weichenthal et al., 2012). Rural populations that engage in farming are particularly vulnerable to the impact of environmental pollutants on their health. Farmers engage in activities necessary for the harvesting of crops or care and feeding of livestock (Blair & Freeman, 2009; Coble et al., 2002; Depczynski & Lower, 2014). These activities require maintenance and repair of mechanical equipment and frequently involve potential exposure to pesticides, fertilizers, engine exhaust fumes, and other hazards (Damalas & Koutroubas, 2016; Sauve et al., 2020; Sauve et al., 2020; Sauve et al., 2020; Sauve et al., 2020). Epidemiological studies with farmers have shown that incidences of common cancers are associated with the farmers' direct exposure to pollutants and chemicals (Blair & Freeman, 2009).
In contrast to common cancers, there are no epidemiological studies on the incidence of bone and joint cancers and their potential link of exposure to occupational hazards. Moreover, information about patients suffering from these neoplasms is not readily accessible (Cronin et al., 2018; Noone et al., 2017). To promote epidemiological research on understudied cancers, this study aimed to: (1) explore the long-term changes in the incidence and mortality rates of bone and joint cancers in the USA over four decades starting from year 1975, (2) determine whether there were gender- and age-specific differences between US patients diagnosed with bone and joint malignancies, and (3) investigate whether there was an association between environmental and demographic factors, such as the residential radon, farmland size, and rural populations and the occurrence of bone and joint cancers in the USA by focusing on the state of Iowa. Iowa was selected for Aim 3 because: (1) It has the largest (71.6%) percentage of homes above the US Environmental Protection Agency (EPA) radon levels of 4pCi/L, (2) according to the US Department of Agriculture (USDA), Iowa has the second largest agricultural industry with more than 86,000 farm operations on 30.6 million acres of farmland, and (3) the Iowa population-based cancer registry provides publicly available county-level incidence and mortality data on cancer cases over a period of at least 40 years.
Methods
US cancer registry data source
The current study used the Surveillance, Epidemiology, and End Results (SEER) database of the National Cancer Institute (NCI)'s Division of Cancer Control and Population Sciences of the USA. SEER collects the cancer patients' information through population-based cancer registries currently covering approximately 48% of the US population (SEER*Explorer, 2021). Each cancer registry is guided by NCI to follow the North American Association of Central Cancer Registries (NAACCR) standards in reporting cancer patient data (https://www.naaccr.org).
Five-year relative cancer survival data source and analysis
The data on the five-year relative survival of patients with bone and joint, lung and bronchus, breast, prostate, and colorectal cancers on the national US level were extracted from SEER9 of NCI that includes 9 cancer registries (San Francisco, Connecticut, Detroit, Hawaii, Iowa, New Mexico, Seattle, Utah, and Atlanta). SEER9 registries contribute cancer cases diagnosed between 1975 and the current data year and cover approximately 9.5% of the US population (Duggan et al., 2016). Other SEERs, accessible via NCI, such as SEER13, SEER18, and SEER21, were not used as those did not collect information about cancer cases from years prior to 1992. The data is presented as a relative survival, which is defined by SEER as the number of observed survivors in a cohort of cancer patients to the number of expected survivors in a comparable cohort of cancer-free individuals (Mariotto et al., 2006).
SEER9 allowed the research team to perform long-term analysis of the 5-year relative survival of patients diagnosed with bone and joint, lung and bronchus, breast, prostate, and colorectal cancers for every ten years between years 1975 and 2014 by finding the mean of the 5-year cancer survival percentage for every 10 years. The data were not separated by gender (except for data from breast and prostate cancer patients), race, ethnicity, or age, and included cases observed within the 9.5% US population.
Long-term trend data source for bone and joint cancers
Information regarding the long-term trends in the incidence and mortality of bone and joint cancer patients on the national US level was extracted from SEER9. All cases reported by the SEER9 registries are classified according to the codes of the International Classification of Diseases in Oncology, third edition (ICD-O-3) (Bernal, 2011). Each registry reported bone and joint cancer patient data by using the following inclusion criteria: (a) primary bone tumor was diagnosed by a recognized medical practitioner, whether clinically or microscopically confirmed, (b) specific stage at diagnosis was localized, regional, or distant (invasive), or the tumor was not staged, (c) the topographical location of the primary tumor was: long or short bones of the upper or lower limb and associated joints (codes C400, C401, C402, C403); overlapping lesion of bones, joints, and articular cartilage of limbs (C408); bones of limbs not otherwise specified (C409); bones of skull, face, and associated joints (C410); mandible and vertebral column (C411, C412); rib, clavicle, and associated joints (C413); pelvic bones and associated joints (C414); overlapping lesion of bones, joints, and articular cartilage (C418); and regional lymph nodes (C419), (d) male and female patients of all ages, race, or ethnicity were included as long as they resided in the geographic area of the registry, and (e) the entered patient age was the one determined at the time of initial diagnosis of the primary bone tumor. Exclusion criteria included: (a) the bone lesion was a result of metastatic growth with a different origin than bone, joint, or articular cartilage (secondary bone cancer), (b) the patient had more than one cancer diagnosis, and (c) the patient had a benign neoplasm.
Methodology used to analyze the long-term trends in bone and joint cancers in the USA
The research team used the SEER* Stat Explorer software (https://seer.cancer.gov/explorer) to access the long-term trends in bone and joint cancers in the USA, which were accessible via SEER9 as indicated above (SEER*Explorer, 2021). Pre-calculated annual age-adjusted incidence rates (AAIR) and age-adjusted mortality rates (AAMR) of patients with bone and joint cancer diagnosis between 1975 and 2014 were extracted from the SEER* Stat Explorer software, separated by gender, and grouped into the following age groups: patients younger than 50 (from birth to 49 years of age), patients between 50 and 64 years of age, and those age 65 and older (65 +). Next, the AAIR and AAMR for each age group were compared over four decades (10-year periods) starting with year 1975 and ending with year 2014. The analysis included all race/ethnicity groups and all stages/histological types of primary bone and joint cancers diagnosed in patients who resided in the 9 cancer registry areas included in SEER9. The cancer cases were not separated by histological subtype or stages as this information was not accessible for the entire period 1975–2014. The rates were per 100,000 and were age-adjusted to the 2000 US standard population (Census P25-1130).
State cancer registry source
The population-based cancer registries at the state level in the USA were accessed through the https://www.cancer-rates.info website. These registries are members of the NCI's SEER program since their inception, and they follow the same standards of reporting cancer patient data as stated above. Each cancer registry provides public access to the number of observed cancer cases along with the AAIR, the AAMR, and the 95% confidence interval (C.I.). The patient data can be further separated by (a) cancer site, (b) geographic location at the level of the entire state or by county, (c) cancer incidence, mortality, or invasiveness, (d) gender, and/or (e) race/ethnicity. However, the cancer-rates.info website provides public access to approximately 30% of the population-based state cancer registries in the USA, which limited the current investigation in undertaking a statewide approach in exploring the long-term trends in bone and joint cancers.
The research team screened all population-based state cancer registries accessible via the cancer-rates.info website for long-term changes in the incidence and mortality rates of patients diagnosed with bone and joint cancers (or leukemia, lymphoma, or all cancer sites). The research team found that the state of Iowa met the authors' criteria for complete reports on cancer cases between years 1975 and 2016 and was selected for further analysis as described next.
Iowa cancer registry data source and analysis
Data on bone and joint cancers, leukemia, lymphoma, or all cancer sites for the state of Iowa were obtained from the Iowa Cancer Registry (https://www.cancer-rates.info/ia/) (Registry, 2021). This population-based cancer registry has served the state of Iowa since 1973 and is a part of the NCI's SEER program (including SEER9). It follows the NCI guidance for reporting cancer patient data including the inclusion and exclusion criteria as defined by ICD-O-3 and stated above. The database provided the number of observed new cases and the AAIR/AAMR for all 99 counties in Iowa from 1975 to 2016.
During the long-term analysis, the bone and joint cancer cases were not separated by race, ethnicity, or geography (county) due to how rare cancers are reported: a minimum of 10 cases are required to calculate a stable age-adjusted rate. The analysis was done by gender and included the mean of AAIR for each 10 years for the entire state of Iowa.
Residential radon data source and analysis
Residential radon data were accessed via the Iowa Department of Public health (IDPH) website (https://idph.iowa.gov/radon/resources) that provides summary of radon test results from private Iowa residences or those obtained during real estate transactions. The tests were conducted by IDPH certified measurement/mitigation specialists from 2010 to 2019 for the entire state of Iowa. The measurements were limited to the radon gas captured by testing devices used in private households or rental properties excluding other locations or other natural resources of radon, such as uranium and granite outside of the residential properties. The measurements did not take into consideration whether the residency was in rural or urban areas of the corresponding county, nor they considered the seasonal changes in the radon levels but were performed before and after a mitigation system was in place.
The research team utilized the unmitigated radon test data for each of the 99 counties in Iowa available from 2010 to 2016. The mean of the radon test measurements (pCi/L) for each county during this period was calculated and used during the Pearson's correlation analysis to compare with the corresponding AAIR of bone and joint cancers for these counties. The study did not utilize radon test results available by zip code and excluded years 2017–2019 to achieve alignment with the available data for bone and joint cancers.
Iowa demographics and regions separation
Information regarding the demographic and housing estimates for the state of Iowa was obtained from the US Census Bureau and corresponds to the 2010 5-year estimate data profile conducted by the American Community Survey (ACS) program (https://www.census.gov/programs-surveys/acs). This information included the Iowa population and household profile, as well as race and age distribution. The research team used the 2010 Census for better alignment with the available data on Iowa cancer patients. Information regarding the dietary behaviors and physical activity of Iowans was obtained from the Center for Disease and Control (CDC)’s Division of Nutrition and Physical activity and Obesity (https://www.cdc.gov/brfss). Information about the average farmland size in acres and the number of farm operations in Iowa was extracted from the Census of Agriculture (https://www.nass.usda.gov), USDA, and the US Census Bureau and corresponds to data last collected in 2012. The Census of Agriculture is conducted every five years and defines a farm as any place from which $1,000 or more of agricultural products were produced and sold, or normally would have been sold, during the census year. Rural area is defined as any incorporated place or census-designated place with fewer than 2500 inhabitants that is located outside of an urban area. However, the rural population values from the 2010 Census may differ from earlier or the most recent (2020) census data due to changes in criteria for delineating urban areas or increases in the urbanization of the Iowa population.
The 99 Iowa counties were next separated between six regions previously established by IDPH, the Iowa Health Care Coalition, and the Iowa National Guard as the six Regional Medical Coordination Centers (RMCCs) to track COVID-19. Region 1 comprises counties in central and south Iowa, Region 2 is north, Region 3 is northwest, Region 4 is southwest, Region 5 is southeast, and Region 6 is northeast Iowa. The counties included in the six regions were the basic units for comparison used to explore the correlation between the AAIR of bone and joint cancers and the rural population or farmland size of Iowa.
Statistics
The AAMR and AAIR of bone and joint cancers during a given 10-year period were calculated by gender and age groups along with the 95% C.I. The mean of AAMR and AAIR of each period was compared to the mean of baseline years 1975–1984. Using a t-test, we determined whether there was a significant difference between the means of two periods. Analyses were conducted using SAS 9.4 (SAS Institute, Cary, NC, USA).
Pearson's correlation coefficient (R) (Mukaka, 2012) was used to measure the strength and direction of the relationship between the AAIR of bone and joint cancers for years 2010–2016 and the following variables: (a) the mean of the unmitigated residential radon levels recorded between 2010 and 2016, (b) the percentage of rural Iowa population, or (c) the average farmland size by county in Iowa. Similar correlation analysis was performed for lymphoma, leukemia, or all cancer sites. The correlation was on the entire state level including all 99 Iowa counties or by region comprising six different regions across Iowa (bone and joint cancers only). The analysis included both genders, all race, ethnicity, and age groups. The significance level was set at P < 0.05.
Results
Long-term analysis of the 5-year survival and mortality rates of patients with bone and joint cancers
Long-term comparison of US patients with bone and joint cancers has revealed that the 5-year survival rate of these patients significantly improved from 52.3% for years 1975–1984 to 69.3% for years 2005–2014 (Fig. 1). This was consistent with the significant decline in the mortality rates among all age groups, males and females, in the USA, compared to baseline years 1975–1984 (Tables 1 and 2). While less people were estimated by SEER-NCI to die from bone and joint cancers compared to patients diagnosed with lung and bronchus, breast, prostate, and colorectal cancers (SEER*Explorer, 2021), the 5-year survival of bone and joint cancer patients was similar to that of patients with colorectal cancers and was significantly lower than that of patients with breast and prostate cancers (Fig. 1).
Comparison of the 5-year survival of patients with bone and joint cancers and those diagnosed with lung and bronchus, colorectal, breast, and prostate cancers. The results are the mean ± SE of the estimated percentages of the 5-year survival rates for every ten years. *P ≤ 0.05 as compared to baseline years 1975–1984 for patients with bone and joint cancers. **P ≤ 0.05 as compared to patients with bone and joint cancers for years 2005–2014
Long-term analysis of the incidence rates of bone and joint cancers by gender and age groups
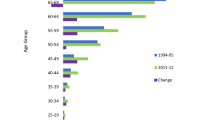
The analysis carried out in the current study showed that the incidence rates of bone and joint cancers did not follow the same trend of improvement as the 5-year survival rates of US patients diagnosed with these cancers. This was particularly alarming for female and male US patients younger than 50 years of age. The mean AAIR within this age group steadily increased over time (Tables 1 and 2). The same age group from around the world showed a similar trend (Online resources 1 and 2). For the rest of the age groups (50–64, and 65 +) in the USA, the mean AAIR declined over time (Tables 1 and 2). The findings demonstrated that females and males, age 49 or younger, appeared most vulnerable to become newly diagnosed with bone and joint cancers in the USA and across the world.
Long-term analysis of the incidence rates of bone and joint cancers by gender in Iowa
The long-term analysis revealed increases in the reported new cases of bone and joint cancers among females in Iowa during the last three decades in the study, when each of those decades was compared to the baseline years 1975–1984. During years 2005–2014, Iowa showed a significant increase in the mean AAIR of bone and joint cancers among female patients (Table 3). A steady increase in new bone and joint cancer cases among male patients was evident during the last two decades in the study. However, these changes were not statistically significant (Table 3).
Correlation between the incidences of bone and joint cancers and the residential radon exposure of Iowans
To explore the increased prevalence of cases of bone and joint cancers in Iowa during 2005–2014, we investigated how exposure to environmental health hazards may have correlated with the observed increases in these cancers for the entire state or by region following the Iowa Department of Public Health (IDPH) RMCC system (See Methods, Fig. 2, and Online resource 3). We asked whether residential exposure to radon in private households was positively associated with the observed increases in bone and joint cancer cases among residents of the corresponding counties. According to Fig. 3a, there was a weak negative correlation (R = − 0.038, P < 0.7) between the mean AAIR of bone and joint cancers and the unmitigated residential radon levels for the entire state of Iowa (all 99 counties included). Similarly, a weak inverse correlation (R = − 0.131, P < 0.19) was recorded for all cancer sites when compared to the residential radon levels in entire state (Fig. 3a).
According to Fig. 3b, four out of six Iowa regions demonstrated inverse correlation between the mean AAIR of bone and joint cancers and the unmitigated radon levels reported for residencies situated in counties within those regions. None of the correlations reached statistical significance with P value < 0.05. Regions 3 and 6 demonstrated a positive correlation, however this was not statistically significant (P < 0.72 and P < 0.76, respectively) (Fig. 3b). In conclusion, our correlation analysis did not show significant positive associations between the increased AAIR of bone and joint cancers and the high residential radon levels at state level or by region in Iowa.
Pearson’s correlation analysis of the association between the AAIR of bone and joint cancers, leukemia, lymphoma, or all cancer sites, and the percentage of rural Iowa population. In a, the association was for all 99 counties taken together. In b, the correlation was by region and for bone and joint cancers only. The AAIR of cancer cases was from 2010 to 2016, while the percentage rural population was calculated based on the 2010 US Census
Comparison of the demographic and agricultural characteristics of Iowa by region
Comparison of the demographic and agricultural characteristics of Iowa revealed that 76.1% of Iowans were 18 years of age or older and 91.9% of the population of Iowa identified as white. Around 36.3% of Iowans resided in rural areas and the average household size was 2.41 people (Table 4).
Comparison by region showed that the largest region (Region 1) comprised the biggest number of farm operations (21,602), but only 28.1% of the population in Region 1 was rural (Table 5). Regions 2–4 were smaller in size and had less farm operations than Region 1, respectively. However, the rural populations in these regions spanned between 47 and 58.4%. Regions 5 and 6 had the smallest average farmland size (between 284 ± 29 and 294 ± 57) and the percentage of rural population was between 33 and 34%. For all regions, most of the population identified as white, the average median age was between 41 and 44 years, and the average household size was approximately 2.4 people (Table 5).
Correlation between the incidences of bone and joint cancers and the rural population of Iowa
Comparative analysis of the AAIR of bone and joint cancers and the percentage rural population within all 99 counties in Iowa revealed a positive correlation between patients with bone cancers and rural area residents (Fig. 4a, R = 0.222, P < 0.02). Because states with a well-developed agriculture may have increased incidences of leukemia and lymphoma (Blair & Freeman, 2009), we next correlated the AAIR of leukemia and lymphoma, or all cancer sites, with the percentage rural Iowa population (Fig. 4a). While there was a positive association between the mean AAIR of leukemia and the percentage rural population (R = 0.189, P < 0.06), the mean AAIR for lymphoma (R = − 0.026, P < 0.80), or all cancer sites (R = − 0.115, P < 0.25) did not positively correlate with the percentage rural population (Fig. 4a).
Further analysis by region demonstrated that 4 Iowa regions had a positive association between the AAIR of bone and joint cancers and the percentage of rural population. Of those 4 regions, the results for Region 1 (central and south Iowa) (R = 0.413, P < 0.04) and Region 3 (northwest Iowa) (R = 0.510, P < 0.02) were statistically significant (Fig. 4b). For Region 2 (north Iowa) (R = 0.025, P < 0.93) and Region 4 (southwest Iowa) (R = 0.403, P < 0.19), the P value was not significant (Fig. 4b). Thus, the mean AAIR of bone and joint cancers between years 2010–2016 appeared higher among Iowans who lived in rural areas; however, this was not evenly distributed between the different regions.
Correlation between the incidences of bone and joint cancers and the average farmland size in Iowa
Positive associations with statistical significance were observed when the AAIR of bone and joint cancers for years 2010–2016 was correlated with the average farmland size in Iowa for all 99 counties (Fig. 5a, R = 0.236, P < 0.02). Similar results were obtained when leukemia cases were correlated with the average farmland size, while lymphoma or all cancer sites showed a weak positive association without statistical significance (Fig. 5a).
Pearson’s correlation analysis of the association between the AAIR of bone and joint cancers, leukemia, lymphoma, or all cancer sites, and the average size of farmland in Iowa. In a, the association was for all 99 counties taken together. In b, the correlation was by region and for bone and join cancers only. The AAIR of cancer cases was from 2010 to 2016, while the average size of the farmland was
Regional correlation, between the mean AAIR of bone and joint cancers for years 2010–2016 and the average farmland size, showed that four regions had positive, while two regions had inverse correlations. None of these correlations were statistically significant (Fig. 5b). Thus, the AAIR of bone and joint cancers between years 2010–2016 positively associated with the average farmland size in Iowa; however, this was not evenly distributed between the different regions.
Discussion
The long-term trends in bone and joint malignancies, investigated in the current study, demonstrated that male and female US patients under the age of 50 were more commonly diagnosed with bone and joint cancers compared to older individuals. The increases in these cancer cases positively correlated with the rural population and the farmland size, but not with the high residential radon levels, as illustrated with the population of Iowa.
During this investigation, we found that the incidences of bone and joint cancers in the USA during 2005–2014 have substantially increased for individuals under age 50 when compared to cases reported back in 1975–1984. The finding that younger individuals were more commonly diagnosed with primary bone and joint cancers is alarming and calls for enhanced scientific research on these cancers. The higher incidence rates of bone and joint cancers in children may coincide with pubertal growth spurt, while the cases observed in older individuals may coincide with Paget's disease (Deshpande et al., 2007; Deyrup et al., 2007). Other risk factors are genetic predisposition to taller statue or heritable cancer syndromes, such as Li-Fraumeni syndrome (Zhang et al., 2018). Gaining a better understanding of genetic factors and signaling pathways that contribute to the oncogenic transformation of bone and cartilage cells could lead to the discovery of novel molecular markers for the prevention of bone and joint tumors. Unfortunately, such approaches could be challenging because bone and joint tumors are rare and clinically relevant findings often present with overlapping morphologies (Lam et al., 2019). Moreover, the population-based cancer registries that collect information from cancer patients do not separate those neoplasms by histological subtypes. Instead, those malignancies are combined in the category “bone and joint” cancers. This, in addition to the fact that bone and joint cancers are understudied, is a practical concern that leaves patients, clinicians, and policy makers with limited information (Gallicchio et al., 2021; Greenlee et al., 2010).
In the current study, Iowa was identified as a state with increasing incidences of bone and joint cancers over the years. This was particularly worrisome for years 2005–2014 as the mean AAIR of bone and joint cancers in Iowa was the highest for both genders, all age groups combined. Iowa is one of the US states with the highest average concentrations of indoor radon. Around 71.6% of Iowan homes have radon concentrations greater than 4 pCi/L. In contrast, the national US average of radon in homes is 1.3pCi/L (Bain et al., 2016; Lantz et al., 2013). Radon can concentrate within the lower levels of buildings becoming potentially hazardous health issue (Stanley et al., 2019). In addition to tobacco smoking, radon is considered a carcinogen linked to the development of lung cancer (Lantz et al., 2013). Population-based case–control study conducted on Iowa women that examined the relationship between cumulative radon exposure and lung cancer suggested a significant lung cancer risk associated with the radon exposure (Field et al., 2000). Similarly, an association between radon levels and the incidence of brain and spinal cord cancers was found to be positive for Iowa but not New Jersey, Wisconsin, Minnesota, or Pennsylvania (Monastero & Meliker, 2020). The Pearson's correlation analysis performed in the current study did not show a positive association between the mean AAIR of bone and joint cancers and the residential exposure to radon from 2010 to 2016 in Iowa. In fact, the correlation was negative, although not statistically significant for four out of the six Iowa regions evaluated. Although our analysis did not provide individual exposure data, this finding supports studies that argue against the existence of a positive association between higher risk of cancer and increased residential exposure to radon (Luckey, 2006).
Iowa is a state with a well-developed agriculture; however, Iowa faces challenges associated with agricultural exposures to chemical, physical, and biological agents. Meta-analysis of studies on Iowa farmers demonstrated that farmers are at higher risk for developing cancer (Blair & Freeman, 2009; Coble et al., 2002). While the incidence rates of bone and joint cancers were never investigated among Iowa farmers and their exposure to chemical and biological agents, the current study found positive correlations between Iowa patients diagnosed with bone and joint cancers and those who resided in rural areas. Our analysis revealed that the rural population of Iowa was more likely to develop bone and joint cancers as there was a positive correlation between those two variables when all 99 Iowa counties were included. Similar association was observed for patients with leukemia, but not for patients with lymphoma, or all cancer sites, indicating that the rural population might be more vulnerable to develop certain (including bone cancers), but not all cancer types. We also found a positive and statistically significant correlation between the average farmland size and the incidences of bone and joint cancers in Iowa. However, these associations were not evenly distributed between the different regions of Iowa. This might be explained by the wide range of exposures in rural areas that can vary between farms depending upon the type of farm operation. For example, exposure to pesticides used on corn takes a few weeks each year, while pesticide exposure among orchardists is more frequent and spans over a longer time period (Blair & Freeman, 2009). The agricultural Health Cohort review on 28 studies, focused on the pesticide exposure and cancer incidence, concluded that of 32 different pesticides, 19 were significantly associated with at least one type of cancer (Weichenthal et al., 2010, 2012). Among them, alachlor, carbaryl, metolachlor, pendimethalin, permethrin, and trifluralin were considered carcinogenic for lymphohematopoietic cancers, melanoma, lung, pancreatic cancer, or colorectal cancer (Acquavella et al., 2004; Alavanja et al., 2004; Andreotti et al., 2009; Hou et al., 2006; Kang et al., 2008). Whether the occurrence of bone and joint cancers may increase among agricultural workers upon use of pesticides is currently unknown.
Other US states may also have increased cases of bone and joint cancers in the most recent decades; however, the bone cancer registries were incomplete or inaccessible via the SEER program of NCI or the CDC cancer statistics database. In fact, in many locations, bone and joint cancers were underreported because a minimum of 10 cases are required to calculate a stable age-adjusted rate. In contrast, the most common cancer sites were well represented by these recourses. Similarly, epidemiological studies and genomic resources were largely focused on common cancers, while rare cancers were studied to lesser extent (Gallicchio et al., 2021).
Due to the rarity of bone and joint malignancies, this investigation did not provide an individual-level exposure evidence that people in rural areas are at direct risk of developing these cancers. Moreover, there might likely be other factors that contributed to the increased incidences of bone and joint cancers but were not evaluated in this study. Risk factors, such as smoking, arsenic, or pesticides were not evaluated. This study did not take into consideration bone fractures, or the impact of race/ethnicity, dietary behaviors, lack of physical exercise or genetic causes because such information is not readily accessible on county level, or available in the public health domains. Due to similar reasons, the investigation was not able to distinguish between the different histological subtypes of bone and joint cancers by age in the in long term.
Conclusions
The long-term trends in bone and joint cancers from 1975 to 2014 signified that male and female patients under the age of 50 were more commonly diagnosed with these cancers compared to older individuals. Some bone sarcomas can be highly lethal but because they do not affect a large portion of the population, they are understudied and thus overlooked in standard reports, including epidemiological studies. According to this investigation, exposure to rural areas and possibly farming, but not to residential radon, as illustrated with the population of Iowa, positively correlated with the increased incidences in bone and joint cancers. This retrospective correlative analysis calls for greater emphasis on bone and joint cancers and recommends to the national representative institutions to: (1) revise the current policies that limit the number of reported rare cancer cases, (2) provide better access to rare cancer documentation for policy makers, clinicians, and researchers, especially for states with higher agricultural industries and vast rural areas in order to respond to their medical needs, as well as evaluate the impact of environmental factors, (3) develop additional programs that conduct surveys in underrepresented rural areas to gain more information about dietary behaviors, daily activities, and exposure to biological and chemical agents, (4) increase the number of the participating population-based cancer registries of the USA so that more than 30% of the population is represented on county-level, and (5) implement strategies to reduce the disparity between common and rare cancers. The use of improved surveillance and epidemiological data could provide opportunities to better explore the disease etiology of bone and joint cancers and help advance future treatment options and prognosis.
References
Acquavella, J. F., Delzell, E., Cheng, H., Lynch, C. F., & Johnson, G. (2004). Mortality and cancer incidence among alachlor manufacturing workers 1968–99. Occupational and Environmental Medicine, 61(8), 680–685. https://doi.org/10.1136/oem.2003.010934
Alavanja, M. C., Dosemeci, M., Samanic, C., Lubin, J., Lynch, C. F., Knott, C., Barker, J., Hoppin, J. A., Sandler, D. P., Coble, J., Thomas, K., & Blair, A. (2004). Pesticides and lung cancer risk in the agricultural health study cohort. American Journal of Epidemiology, 160(9), 876–885. https://doi.org/10.1093/aje/kwh290
Alexander, D. D., Mink, P. J., Adami, H. O., Chang, E. T., Cole, P., Mandel, J. S., & Trichopoulos, D. (2007). The non-Hodgkin lymphomas: A review of the epidemiologic literature. International Journal of Cancer, 120(Suppl 12), 1–39. https://doi.org/10.1002/ijc.22719
Andreotti, G., Freeman, L. E., Hou, L., Coble, J., Rusiecki, J., Hoppin, J. A., Silverman, D. T., & Alavanja, M. C. (2009). Agricultural pesticide use and pancreatic cancer risk in the agricultural health study Cohort. International Journal of Cancer, 124(10), 2495–2500. https://doi.org/10.1002/ijc.24185
Bain, A. A., Abbott, A. L., & Miller, L. L. (2016). Successes and challenges in implementation of radon control activities in Iowa, 2010–2015. Preventing Chronic Disease, 13, E50. https://doi.org/10.5888/pcd13.150596
Bernal, A. (2011). The blending of ICD-O-3 with SEER inquiry (SINQ). Journal of registry management, 38(4), 212–213.
Blair, A., & Freeman, L. B. (2009). Epidemiologic studies in agricultural populations: Observations and future directions. Journal of Agromedicine, 14(2), 125–131. https://doi.org/10.1080/10599240902779436
Burns, C. J., & Juberg, D. R. (2021). Cancer and occupational exposure to pesticides: An umbrella review. International Archives of Occupational and Environmental Health, 94(5), 945–957. https://doi.org/10.1007/s00420-020-01638-y
Coble, J., Hoppin, J. A., Engel, L., Elci, O. C., Dosemeci, M., Lynch, C. F., & Alavanja, M. (2002). Prevalence of exposure to solvents, metals, grain dust, and other hazards among farmers in the agricultural health study. Journal of Exposure Analysis and Environmental Epidemiology, 12(6), 418–426. https://doi.org/10.1038/sj.jea.7500248
Cohen, S. M., Boobis, A. R., Dellarco, V. L., Doe, J. E., Fenner-Crisp, P. A., Moretto, A., Pastoor, T. P., Schoeny, R. S., Seed, J. G., & Wolf, D. C. (2019). Chemical carcinogenicity revisited 3: Risk assessment of carcinogenic potential based on the current state of knowledge of carcinogenesis in humans. Regulatory Toxicology and Pharmacology, 103, 100–105. https://doi.org/10.1016/j.yrtph.2019.01.017
Cronin, K. A., Lake, A. J., Scott, S., Sherman, R. L., Noone, A. M., Howlader, N., Henley, S. J., Anderson, R. N., Firth, A. U., J, Ma., Kohler, B. A., & Jemal, A. (2018). Annual report to the nation on the status of cancer, part I: National cancer statistics. Cancer, 124(13), 2785–2800. https://doi.org/10.1002/cncr.31551
Dai, X., Ma, W., He, X., & Jha, R. K. (2011). Review of therapeutic strategies for osteosarcoma, chondrosarcoma, and Ewing’s sarcoma. Medical Science Monitor, 17(8), RA177–RA190. https://doi.org/10.12659/MSM.881893
Damalas, C., & Koutroubas, S. (2016). Farmers’ exposure to pesticides: Toxicity types and ways of prevention. Toxics, 4(1), 1. https://doi.org/10.3390/toxics4010001
Damron, T. A., Ward, W. G., & Stewart, A. (2007). Osteosarcoma, chondrosarcoma, and Ewing’s sarcoma: National cancer data base report. Clinical Orthopaedics and Related Research, 459, 40–47. https://doi.org/10.1097/BLO.0b013e318059b8c9
David, E., Blanchard, F., Heymann, M. F., De Pinieux, G., Gouin, F., Redini, F., & Heymann, D. (2011). The bone niche of chondrosarcoma: A sanctuary for drug resistance, tumour growth and also a source of new therapeutic targets. Sarcoma, 2011, 932451. https://doi.org/10.1155/2011/932451
Depczynski, J., & Lower, T. (2014). A review of prostate cancer incidence and mortality studies of farmers and non-farmers, 2002–2013. Cancer Epidemiology, 38(6), 654–662. https://doi.org/10.1016/j.canep.2014.09.001
Deshpande, A. M., Akunowicz, J. D., Reveles, X. T., Patel, B. B., Saria, E. A., Gorlick, R. G., Naylor, S. L., Leach, R. J., & Hansen, M. F. (2007). PHC3, a component of the hPRC-H complex, associates with E2F6 during G0 and is lost in osteosarcoma tumors. Oncogene, 26(12), 1714–1722. https://doi.org/10.1038/sj.onc.1209988
Deyrup, A. T., Montag, A. G., Inwards, C. Y., Xu, Z., Swee, R. G., & Krishnan Unni, K. (2007). Sarcomas arising in paget disease of bone: A clinicopathologic analysis of 70 cases. Archives of Pathology and Laboratory Medicine, 131(6), 942–946. https://doi.org/10.1043/1543-2165(2007)131[942:SAIPDO]2.0.CO;2
Duggan, M. A., Anderson, W. F., Altekruse, S., Penberthy, L., & Sherman, M. E. (2016). The Surveillance, epidemiology, and end results (SEER) program and pathology: Toward strengthening the critical relationship. American Journal of Surgical Pathology, 40(12), e94–e102. https://doi.org/10.1097/PAS.0000000000000749
Field, R. W., Steck, D. J., Smith, B. J., Brus, C. P., Fisher, E. L., Neuberger, J. S., Platz, C. E., Robinson, R. A., Woolson, R. F., & Lynch, C. F. (2000). Residential radon gas exposure and lung cancer: The Iowa radon lung cancer study. American Journal of Epidemiology, 151(11), 1091–1102. https://doi.org/10.1093/oxfordjournals.aje.a010153
Gallicchio, L., Daee, D. L., Rotunno, M., Barajas, R., Fagan, S., Carrick, D. M., Divi, R. L., Filipski, K. K., Freedman, A. N., Gillanders, E. M., Lam, T. K., & Nelson, S. A. (2021). Epidemiologic research of rare cancers: Trends, resources, and challenges. Cancer Epidemiology, Biomarkers & Prevention. https://doi.org/10.1158/1055-9965.EPI-20-1796
Gelderblom, H., Hogendoorn, P. C., Dijkstra, S. D., van Rijswijk, C. S., Krol, A. D., Taminiau, A. H., & Bovee, J. V. (2008). The clinical approach towards chondrosarcoma. The Oncologist, 13(3), 320–329. https://doi.org/10.1634/theoncologist.2007-0237
Greenlee, R. T., Goodman, M. T., Lynch, C. F., Platz, C. E., Havener, L. A., & Howe, H. L. (2010). The occurrence of rare cancers in U.S. Adults, 1995–2004. Public Health Reports, 125(1), 28–43. https://doi.org/10.1177/003335491012500106
Grunewald, T. G. P., Cidre-Aranaz, F., Surdez, D., Tomazou, E. M., de Alava, E., Kovar, H., Sorensen, P. H., Delattre, O., & Dirksen, U. (2018). Ewing sarcoma. Nature Reviews. Disease Primers, 4(1), 5. https://doi.org/10.1038/s41572-018-0003-x
Heare, T., Hensley, M. A., & Dell’Orfano, S. (2009). Bone tumors: osteosarcoma and Ewing’s sarcoma. Current Opinion in Pediatrics, 21(3), 365–372. https://doi.org/10.1097/MOP.0b013e32832b1111
Hou, L., Lee, W. J., Rusiecki, J., Hoppin, J. A., Blair, A., Bonner, M. R., Lubin, J. H., Samanic, C., Sandler, D. P., Dosemeci, M., & Alavanja, M. C. (2006). Pendimethalin exposure and cancer incidence among pesticide applicators. Epidemiology, 17(3), 302–307. https://doi.org/10.1097/01.ede.0000201398.82658.50
Kang, D., Park, S. K., Beane-Freeman, L., Lynch, C. F., Knott, C. E., Sandler, D. P., Hoppin, J. A., Dosemeci, M., Coble, J., Lubin, J., Blair, A., & Alavanja, M. (2008). Cancer incidence among pesticide applicators exposed to trifluralin in the agricultural health study. Environmental Research, 107(2), 271–276. https://doi.org/10.1016/j.envres.2008.01.010
Kim, S. H., Hwang, W. J., Cho, J. S., & Kang, D. R. (2016). Attributable risk of lung cancer deaths due to indoor radon exposure. Ann Occup Environ Med, 28, 8. https://doi.org/10.1186/s40557-016-0093-4
Koff, J. L., Chihara, D., Phan, A., Nastoupil, L. J., Williams, J. N., & Flowers, C. R. (2015). To each its own: Linking the biology and epidemiology of NHL subtypes. Current Hematologic Malignancy Reports, 10(3), 244–255. https://doi.org/10.1007/s11899-015-0267-0
Lam, S. W., van IJzendoorn, D. G., Cleton-Jansen, A. M., Szuhai, K., & Bovee, J. (2019). Molecular pathology of bone tumors. J Mol Diagn, 21(2), 171–182. https://doi.org/10.1016/j.jmoldx.2018.11.002
Lantz, P. M., Mendez, D., & Philbert, M. A. (2013). Radon, smoking, and lung cancer: The need to refocus radon control policy. American Journal of Public Health, 103(3), 443–447. https://doi.org/10.2105/AJPH.2012.300926
Luckey, T. D. (2006). Radiation hormesis: The good, the bad, and the ugly. Dose Response, 4(3), 169–190. https://doi.org/10.2203/dose-response.06-102.Luckey
Mariotto, A. B., Wesley, M. N., Cronin, K. A., Johnson, K. A., & Feuer, E. J. (2006). Estimates of long-term survival for newly diagnosed cancer patients: A projection approach. Cancer, 106(9), 2039–2050. https://doi.org/10.1002/cncr.21803
Monastero, R. N., & Meliker, J. R. (2020). Incidence of brain and spinal cord cancer and county-level radon levels in New Jersey, Wisconsin, Minnesota, Pennsylvania, and Iowa, USA. Environmental Geochemistry and Health, 42(2), 389–395. https://doi.org/10.1007/s10653-019-00368-6
Mukaka, M. M. (2012). Statistics corner: A guide to appropriate use of correlation coefficient in medical research. Malawi Medical Journal, 24(3), 69–71.
Noone, A. M., Cronin, K. A., Altekruse, S. F., Howlader, N., Lewis, D. R., Petkov, V. I., & Penberthy, L. (2017). Cancer incidence and survival trends by subtype using data from the surveillance epidemiology and end results program, 1992–2013. Cancer Epidemiology, Biomarkers & Prevention, 26(4), 632–641. https://doi.org/10.1158/1055-9965.EPI-16-0520
Otoukesh, B., Boddouhi, B., Moghtadaei, M., Kaghazian, P., & Kaghazian, M. (2018). Novel molecular insights and new therapeutic strategies in osteosarcoma. Cancer Cell International, 18, 158. https://doi.org/10.1186/s12935-018-0654-4
Ottaviani, G., & Jaffe, N. (2009). The epidemiology of osteosarcoma. Cancer Treatment and Research, 152, 3–13. https://doi.org/10.1007/978-1-4419-0284-9_1
Patel, D. M., Jones, R. R., Booth, B. J., Olsson, A. C., Kromhout, H., & Straif, K. (2020). Parental occupational exposure to pesticides, animals and organic dust and risk of childhood leukemia and central nervous system tumors: Findings from the international childhood cancer cohort consortium (I4C). International Journal of Cancer, 146(4), 943–952. https://doi.org/10.1002/ijc.32388
Rathore, R., & Van Tine, B. A. (2021). Pathogenesis and current treatment of osteosarcoma: Perspectives for future therapies. Journal of Clinical Medicine, 10(6), 1182. https://doi.org/10.3390/jcm10061182
Registry, I. C. (2021, 1 Oct 2021). Age-Adjusted Cancer Incidence Rates by County in Iowa, All Sites, 2014–2018. Iowa Cancer Registry. Retrieved 16 Dec 2021 from http://cancer-rates.info/ia
Sauve, J. F., Locke, S. J., Josse, P. R., Stapleton, E. M., Metwali, N., Altmaier, R. W., Andreotti, G., Thorne, P. S., Hofmann, J. N., Freeman, L. E., & Friesen, M. C. (2020a). Characterization of inhalable endotoxin, glucan, and dust exposures in Iowa farmers. International Journal of Hygiene and Environmental Health, 228, 113525. https://doi.org/10.1016/j.ijheh.2020.113525
Sauve, J. F., Stapleton, E. M., O’Shaughnessy, P. T., Locke, S. J., Josse, P. R., Altmaier, R. W., Silverman, D. T., Paul, D. L., Albert, S., Freeman, L. E. B., Hofmann, J. N., Thorne, P. S., Jones, R. R., & Friesen, M. C. (2020b). diesel exhaust exposure during farming activities: Statistical modeling of continuous black carbon concentrations. Annals of Work Exposures and Health, 64(5), 503–513. https://doi.org/10.1093/annweh/wxaa032
SEER*Explorer (2021). An interactive website for SEER cancer statistics [Internet]. Surveillance Research Program, National Cancer Institute. [Cited 2021 April 15]. Available from https://seer.cancer.gov/explorer/
Siegel, R. L., Miller, K. D., & Jemal, A. (2020). Cancer statistics, 2020. CA: A Cancer Journal for Clinicians, 70(1), 7–30. https://doi.org/10.3322/caac.21590
Stanley, F. K. T., Irvine, J. L., Jacques, W. R., Salgia, S. R., Innes, D. G., Winquist, B. D., Torr, D., Brenner, D. R., & Goodarzi, A. A. (2019). Radon exposure is rising steadily within the modern North American residential environment, and is increasingly uniform across seasons. Science and Reports, 9(1), 18472. https://doi.org/10.1038/s41598-019-54891-8
Weichenthal, S., Moase, C., & Chan, P. (2010). A review of pesticide exposure and cancer incidence in the agricultural health study cohort. Environmental Health Perspectives, 118(8), 1117–1125. https://doi.org/10.1289/ehp.0901731
Weichenthal, S., Moase, C., & Chan, P. (2012). A review of pesticide exposure and cancer incidence in the agricultural health study cohort. Ciência & Saúde Coletiva, 17(1), 255–270. https://doi.org/10.1590/s1413-81232012000100028
Zamborsky, R., Kokavec, M., Harsanyi, S., & Danisovic, L. (2019). Identification of prognostic and predictive osteosarcoma biomarkers. Medical Sciences, 7(2), 28. https://doi.org/10.3390/medsci7020028
Zhang, C., Morimoto, L. M., de Smith, A. J., Hansen, H. M., Gonzalez-Maya, J., Endicott, A. A., Smirnov, I. V., Metayer, C., Wei, Q., Eward, W. C., Wiemels, J. L., & Walsh, K. M. (2018). Genetic determinants of childhood and adult height associated with osteosarcoma risk. Cancer, 124(18), 3742–3752. https://doi.org/10.1002/cncr.31645
Acknowledgements
We thank Aldijana Dizdarevic, May Moreland, Josh Waltman, Wachie Peters, Mikayla Schnurr, Haley Wood, Ryan McClure, and Ahren Reitsma, former students in the Waukee APEX program, for their involvement in the project “raising awareness of bone and joint cancers in Iowa,” which was the foundation for the development of this manuscript.
Funding
No funding was received for conducting this study.
Author information
Authors and Affiliations
Contributions
JDN, HAS, DY, DL, and EAA designed and directed the investigation. EAA wrote the manuscript and performed the Pearson's correlation analysis. JDN collected and analyzed the bone and joint cancer data on the national (USA), as well as the state level. BDW collected and analyzed the radon data for the state of Iowa. RAB conducted the 5-year survival rate analysis and statistics for the USA. MPB collected and analyzed the data on bone and joint cancers around the world. DY and DL conducted the bone and joint cancer statistics for the USA, the world, and the states of Iowa. All authors participated in data interpretation, reviewed, and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no potential conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nilles, J.D., Lim, D., Boyer, M.P. et al. The occurrence of bone and joint cancers and their association with rural living and radon exposure in Iowa. Environ Geochem Health 45, 925–940 (2023). https://doi.org/10.1007/s10653-022-01261-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-022-01261-5