Abstract
The aim of this study was to assess the effects of air pollutants on hospital admissions for respiratory disease (RD) by using distributed lag nonlinear model (DLNM) in Lanzhou during 2014–2019. In this study, the dataset of air pollutants, meteorological, and daily hospital admissions for RD in Lanzhou, from January 1st, 2014 to December 31st, 2019, were collected from three national environmental monitoring stations, China meteorological data service center, and three large general hospitals, respectively. A time-series analysis with DLNM was used to estimate the associations between air pollutants and hospital admissions for RD including the stratified analysis of age, gender, and season. The key findings were expressed as the relative risk (RR) with a 95% confidence interval (CI) for single-day and cumulative lag effects (0–7). A total of 90, 942 RD hospitalization cases were identified during the study period. The highest association (RR, 95% CI) of hospital admissions for RD and PM2.5 (1.030, 1.012–1.049), and PM10 (1.009, 1.001–1.015), and NO2 (1.047, 1.024–1.071) were observed at lag 07 for an increase of 10 μg/m3 in the concentrations, and CO at lag07 (1.140, 1.052–1.236) for an increase of 1 mg/m3 in the concentration. We observed that the RR estimates for gaseous pollutants (e.g., CO and NO2) were larger than those of particulate matter (e.g., PM2.5 and PM10). The harmful effects of PM2.5, PM10, NO2, and CO were greater in male, people aged 0–14 group and in the cold season. However, no significant association was observed for SO2, O38h, and total hospital admissions for RD. Therefore, some effective intervention strategies should be taken to strengthen the treatment of the ambient air pollutants, especially gaseous pollutants (e.g., CO and NO2), thereby, reducing the burden of respiratory diseases.
Similar content being viewed by others
Introduction
With the rapid economic development occurring in the past 3 decades in China, the level of air pollution has increased from various emission sources. As the system with the most frequent and extensive contact between the human body and the external environment, the respiratory system will inhale a certain amount of oxygen during outdoor air exchange. Air pollutants of particulate matter (PM), organic compounds, and gaseous air pollutants such as sulfur dioxide (SO2), nitrogen dioxide (NO2), ozone (O3), and CO (carbon monoxide) can be carriers of small viruses and bacteria as well as being sensitinogen for respiratory diseases (RD) development (Cui et al., 2018). And these pollutants have irritant effects that can induce cough reflex hypersensitivity, dyspnea, and bronchial hyper-responsiveness (Fang et al., 2019). Hence, the respiratory system is the main organ affected by air pollution. Numerous epidemiological studies have indicated that air pollutants such as PM, SO2, NO2, O3, and CO are responsible for increasing respiratory morbidity in different populations around the world, especially from the megacities (Çapraz et al., 2017; Chang et al., 2020; Luo et al., 2018; Phosri et al., 2019; Phung et al., 2016; Qiu et al., 2018; Sofwan et al., 2021; Vahedian et al., 2017). For example, a study conducted by Phung et al. (2016) reported effect estimates (relative risks, RRs) of 1.007 (95% CI 1.002, 1.013), 1.020 (95% CI 1.010, 1.030), and 1.080 (95% CI 1.060, 1.011) for each 10 μg/m3 increment of PM10, SO2, and NO2, respectively. A study in Arak, Iran reported that a 10 μg/m3 increase in PM2.5, PM10, SO2, NO2, O3, and 1 mg/m3 in CO resulted RRs of 1.010 (95% CI 0.990, 1.030), 1.010 (95% CI 1.004, 1.017), 1.010 (95% CI 0.998, 1.020), 1.032 (95% CI 1.003, 1.060), 0.975 (95% CI 0.960, 0.990), and 1.090 (95% CI 1.040, 1.140), respectively, for respiratory admission (Vahedian et al., 2017). For Istanbul, Turkey, it was reported that an increase of 10 μg/m3 in concentrations of PM2.5 and NO2 over 4 days of lag corresponded to RR = 1.015 (95% CI 1.011–1.020) and RR = 1.013 (95% CI 1.010–1.015) increase of respiratory admission, respectively (Çapraz et al., 2017). Luo et al. (2018) applied a time-series study to evaluate the association between air pollution and RD hospitalization in Taiyuan. The results showed that an increase of 10 μg/m3 in the levels of PM2.5, PM10, SO2, and NO2 had a RR of 1.005 (95% CI 1.001–1.010), 1.004 (95% CI 1.001–1.008), 1.008 (95% CI 1.003–1.012), and 1.027 (95% CI 1.010–1.044) for respiratory morbidity. Another study including 17 cities in Sichuan, China reported that per 10 μg/m3 increase of PM2.5, PM10, SO2, and NO2 corresponded to a RR for 1.005 (95% CI 1.004, 1.007), 1.004 (95% CI 1.003, 1.005), 1.025 (95% CI 1.015, 1.036), and 1.024 (95% CI 1.018, 1.030) increases in overall respiratory admission (Qiu et al., 2018). Phosri et al. (2019) conducted Poisson time-series regression model to explore the relationship between urban air pollutants and hospital admissions for RD in Bangkok, Thailand, and showed that each 10 μg/m3 increase of PM10, NO2, SO2, O3 and 1 mg/m3 in CO was associated with a RR of 1.012 (95% CI 1.008–1.016), 1.014 (95% CI 1.010–1.019), 1.054 (95% CI 1.022–1.068), 1.007 (95% CI 1.002–1.012), and 1.077 (95% CI 1.052–1.102) for respiratory admissions. And in Shenyang, a time-series study found that per 10 μg/m3 increase in PM2.5, PM10, NO2, SO2, O3, and 1 mg/m3 in CO concentrations were associated with a RR of 1.003 (95% CI 0.998–1.007), 1.009 (95% CI 1.005–1.013), 1.041 (95% CI 1.035–1.048), 1.007 (95% CI 1.001–1.014), 0.989(95% CI 0.976, 0.993), and 1.021 (95% CI 1.016–1.025) for respiratory admissions, respectively (Chang et al., 2020). In Kuala Lumpur, an epidemiological study of the health consequences due to air pollution exposures concluded that respiratory morbidity in the local residents was significantly associated with PM10 (RR: 1.055; 95% CI 1.013, 1.098) (per 10 μg/m3 increment), SO2 (RR: 0.993; 95% CI 0.931, 1.059) (per 10 μg/m3 increment), NO2 (RR: 1.100; 95% CI 1.014, 1.93) (per 10 μg/m3 increment), and CO (RR: 1.116; 95% CI 1.016, 1.226) exposure (per 1 mg/m3 increment) (Sofwan et al., 2021).
Overall, studies mentioned above mainly discussed the effects of different ambient air pollutants on risk of respiratory disease in megacities including Ho Chi Minh City (Phung et al., 2016), Istanbul (Çapraz et al., 2017), Bangkok (Phosri et al., 2019), Kuala Lumpur (Sofwan et al., 2021), as well as in the Chinese cities of Taiyuan (Luo et al., 2018), Shenyang (Chang et al., 2020), and Sichuan (Qiu et al., 2018), and less research was available in the arid inland cities of northwest China, where the components of pollution, climate condition, and socio-demographic status (i.e., disease pattern and socioeconomic characteristic) differ from those cites. In addition, there are still some insufficiency in current researches. First, limit study of the health effects of air pollution among adult residents has been performed (Chang et al., 2020; Phosri et al., 2019; Qiu et al., 2018). Despite not being a vulnerable group, adults are generally exposed to higher levels of air pollution, particularly from vehicles, due to work-related travel activities. Second, there are few analogous researches assessing the relationship from the warm and cold season prospective for different air pollutants. Meanwhile, there are no similar time-series studies reporting statistical association between O3 (maximum 8-h moving average) and CO exposure, lag effect, and the RD morbidity. Third, time-series analysis based on the ordinary Poisson regression model which mainly focuses on the correlational relationship is widely used in current researches. These models can only observe the average risk estimates of RD morbidity related to exposure to air pollution during a single-exposure time window. In recent years, a distributed lag nonlinear model (DLNM), which attracted much attention from scholars, has revealed an exposure–response relationship, as well as a lag-response relationship from the lag dimension, which indicated the duration of lag effects (Sofwan et al., 2021; Xie et al., 2019; Xu et al., 2021). To avoid the limitations of Poisson regression model, we adopted an advanced method, a distributed lag nonlinear model (DLNM) which can represent both nonlinear exposure–response dependencies and delay effects.
Lanzhou, which is located in the inland northwest China, so the unique basin topography and special meteorological conditions cause air pollutants difficult to diffuse. Some scholars have reported on the air pollution and hospitalization effect or outpatient effect in Lanzhou (Cheng et al., 2021; Dong, Liu, et al., 2021a). However, those studies did not assess the lag-response relationship between daily respiratory hospital admissions and air pollutants as well as the adverse health effects of exposure to CO and O38h or during seasonal variations. Therefore, we aim to explore the relationship between daily exposure to PM2.5, PM10, SO2, NO2, O38h, and CO and the risk of hospitalization for RD in Lanzhou. Subgroup analyses for gender, age, and seasonal periods were also performed to assess their correction effects.
Data and methods
Study area
Lanzhou, the capital city of Gansu Province, is situated in the interior of northwest China (35.57°–37.12° N, 102.59–104.57° E), which has a typical temperate continental climate with four distinct seasons. According to Lanzhou statistical yearbook 2020, the city had 4 main districts with a total area of 1100 km2 and a total permanent population of 3.79 million in 2020. The industry of the city is petrochemical, metallurgical, and mechanical industries, and so on.
Data sources
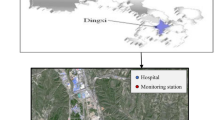
According to hospital admission and geographical locations of Lanzhou urban general hospitals, three large general hospitals with a complete electronic medical record system were selected as the data source. The city territory is located on the slopes of the mountains and descends from the west to the east with urban line stretching along the river from the north to the south for over 40 km (see Fig. S1). Residential areas are mainly distributed in strips from east to west, and these three hospitals are located in the eastern and western areas of the city, which are densely populated areas with convenient transportation. As top three large-scale general hospitals in Lanzhou, the chosen hospitals have a good reputation for treating respiratory diseases with their advanced equipment, complete medical departments, and strong business and technical capabilities. More than 80% of local residents choose these hospitals for seeking medical help (Dong, Liu, et al., 2021a; Dong, Wang, et al., 2021b). To some extent, we think that these hospitals account for most of the admissions by the population. Data on respiratory hospital admissions from January 1, 2014, to December 31, 2019, were obtained from these hospitals. The locations of these hospitals are shown in Fig. 1.
We derived medical records of patients from the information system of each hospital, including gender, age, residential address, date of admission, and principal diagnosis. All cases were coded according to International Classification of Diseases, 10th Revision (ICD-10). The respiratory diseases’ data (ICD-10 codes J00-J99) were selected and targeted in the database. Exclusion criteria: (1) Patients whose residential address is outside of the four urban districts in Lanzhou; (2) those who stay in Lanzhou for less than 6 months; and (3) records with incomplete information. Finally, hospital admissions for respiratory diseases visits (ICD-10 codes J00-J99) were identified as health outcomes. In addition, hospital admissions for different gender (male and female), and different age groups (0–14 years, 15–64 years, and ≥ 65 years old) were also separately analyzed. There were 90,942 admissions for RD during 2014–2019, including 53,549 person times of male, 37,393 person times of female, 29,210 person times of people 0–14 years old, 27,406 person times of people 14–64 years old, and 34,326 person times of people ≥ 65 years old.
Hourly data on air pollution, including PM2.5, PM10, SO2, NO2, CO, and O3, were obtained from three national environmental monitoring stations in the city of Lanzhou. These monitoring stations are required to be located away from pollution sources according to the national technical guidelines, and monitoring of air pollution was done in accordance with mandatory quality assurance/quality control (QA/QC) procedures set by the Ministry of Ecological Environment in China, ensuring the quality of automatic environmental air monitoring data. The three stations are concentrated in the urban area of Lanzhou (their locations are shown in Fig. 1). Since Lanzhou is located at a narrow, long, NW–SE-oriented valley basin, and the distance from east to west and from north to south of Lanzhou are about 40 km and 3–8 km, respectively, the urban area is small. Therefore, the three hospitals and the three monitoring stations are within 5–15 km of one another, and those data can effectively reflect the situation of hospitalization of respiratory diseases and the average air pollution exposure throughout the entire city of Lanzhou. However, because the addresses of the patients who came to get treatment were recorded without detail and standardization from the three hospitals, the individuals’ residence addresses information could not be converted into the corresponding latitude and longitude coordinates. Therefore, we were not able to use spatial interpolation or pollution data from the nearest air quality monitoring station to reflect the exposure level of the hospital population (Dong, Wang, et al., 2021b). After consulting the relevant literature (Chang et al., 2020; Phosri et al., 2019; Sofwan et al., 2021), daily 24 h concentrations were calculated from hourly data taken at the 3 monitoring stations for all five pollutants except O3, which were calculated as 8 h maximum values (from 10 a.m. to 6 p.m.).
The daily meteorological data near the ground of Lanzhou were obtained from China Meteorological Science Data Sharing Service Network, including daily average temperature (℃) and daily average relative humidity (%).
Statistical analysis
Since daily respiratory hospital admissions approximate an over dispersed Poisson distribution, and the relationship between morbidity and air pollutants is likely nonlinear. Therefore, a quasi-Poisson distribution with DLNM was used to explore the non-linear and delayed effects of air pollutant on the number of respiratory cases. The model is as follows:
where t is the time observed (days); Yt is the dependent variable, number of respiratory admissions at t day; μt is the expected mean of Yt; α is the constant of model; Xt,l represents air pollutant cross basis matrix, β is the coefficient of the matrix; l is the maximum number of days of lag, and we adopt a natural cubic spline (ns) function for air pollutant and a polynomial function for lag; Timet is the time variable, day t, used to control the long-term trend and seasonality of time; df is the degree of freedom; Temt is the average temperature on day t; and RHt is the average relative humidity of the day t; Dow is the day of the week effect; and Hol is the day of the holiday effect. All degrees of freedom in the model were selected using Akaike Information Criterion for quasi-Poisson (Q-AIC), and lower the AIC value, better the formula. For air pollutant, we used an ns function to estimate the lag space with 3 internal knots placed at equally spaced values on the log scale and polynomial transformations with 3 df. To control for the long-term trends and seasonality, we used an ns with 7 df per year of data for the variable Timet. Meanwhile, a natural spline with df = 3 was also used in order to control temperature and relative humidity.
Some studies have reported a lag effect of air pollution on respiratory admissions, which is usually under seven days (Chang et al., 2020; Chen et al., 2010; Qiu et al., 2018; Vahedian et al., 2017). Therefore, the maximum lag time after exposure to air pollutants was set to 7 days herein. To effectively depict the lagged effects of air pollutant on respiratory admissions, we calculated the single-day lag influence (lag 1, lag2, lag3, lag4, lag5, lag6, and lag7) and the cumulative lag influence (lag01, lag02, lag03, lag04, lag05, lag06, and lag07). Further stratification analysis was performed by the gender (male and female), age (0–14 years, 15–64 years and ≥ 65 years old), hospital (Lanzhou University First Hospital, Lanzhou University Second Hospital, and Gansu Provincial People’s Hospital), and season [warm season (from May to October) and cold season (from November to the next April)]. We chose the lag day with the largest estimated effect in the single-pollutant model to conduct the following sensitivity analysis. (1) Fitting two-pollutant models considering the strong correlation between PM2.5 and PM10 (0.857, Table2), in the following two-pollutant models for PM2.5 and PM 10 were PM excluded. (2) Changing the degrees of freedom (6–10 df) in the ns function of time.
We present the associations with the relative risks (RRs) and 95% confidence intervals (CIs) per 10 μg/m3 increase in the PM2.5, PM10, SO2, NO2, and O3 concentrations and per 1 mg/m3 increase in the level of CO. All the calculations were done using R software version 3.6.3 (R Core Team, 2014), with its “DLNM” package.
Except daily relative humidity, the data of daily temperature, and the data of daily concentrations of PM2.5, PM10, SO2, NO2, CO, and O38h disobeyed normal distribution, so spearman correlation analysis was used to explore the relationship between daily air pollutants and meteorological factors.
Results
Table 1 summarizes the results of the descriptive statistics on hospital admissions with RD, concentration of air pollutants and meteorological factors in Lanzhou City during 2014–2019. The daily mean count of RD hospital admissions was 42 (range, 1–149 visits/day). The daily RD hospital admissions per day for male, female, age 0–14 years, age 15–64 years, and age ≥ 65 years were 24, 17, 13, 12, and 16, respectively. Daily average concentrations were 48.97 μg/m3 for PM2.5, 114.90 μg/m3 for PM10, 21.13 μg/m3 for SO2, 47.36 μg/m3 for NO2, 88.24 μg/m3 for O38h, and 1.24 mg/m3 for CO during the study period. The average concentration of SO2 meets the requirements of the secondary standard (60 μg/m3) in Chinese Ambient Air Quality Standard, and that of PM2.5, PM10, and NO2 all exceed the secondary standards in Chinese Ambient Air Quality Standard (35 μg/m3, 70 μg/m3, and 40 μg/m3, respectively). The daily average temperature and relative humidity were 11.34 ℃ and 51.03%, respectively.
Figure 2 shows the time-series plots of daily RD hospital admissions and air pollutants daily concentration in Lanzhou from January 1, 2014 to December 31, 2019. The daily RD hospital admissions exhibit regular seasonal fluctuation, and RD hospital admissions in cold season (55 visits/day) were much higher than in warm season (47 visits/day) (P = 0.000). The concentrations of PM2.5, PM10, SO2, NO2, and CO had similar characteristics of periodic fluctuation, which were higher in the cold season and lower in the warm season. However, the level of O38h showed an inverse trend. The average concentrations of PM2.5 (51.35 μg/m3 vs. 30.29 μg/m3, P = 0.000), PM10 (117.40 μg/m3 vs. 76.53 μg/m3, P = 0.000), SO2 (27.05 μg/m3 vs. 11.36 μg/m3, P = 0.000), NO2 (61.53 μg/m3 vs. 43.42 μg/m3, P = 0.000), and CO (1.45 mg/m3 vs. 0.74 mg/m3, P = 0.000) in cold season were higher than those in warm season except for O38h (78.92 μg/m3 vs. 117.99 μg/m3, P = 0.000).
Table 2 shows the correlation between air pollutants and meteorological factors through the Spearman rank correlation coefficient. In the following, “r” is used to represent the correlation coefficient. Daily PM2.5, PM10, SO2, NO2, and CO concentrations were significantly positively correlated with each other (r ranged from 0.435 to 0.857), among which the highest significant correlation was found between PM2.5 and PM10 (r = 0.857), followed by SO2 and CO (r = 0.799). O38h was weakly and negatively correlated to other pollutants (r ranged from − 0.483 to − 0.044). All air pollutants were negatively correlated with temperature, except for O38h (r = 0.643), and all air pollutants were negatively correlated with relative humidity, except for CO (r = − 0.038, P > 0.05).
Figure 3 shows the three-dimensional plots of relative risks (RRs) of RD admissions along single-pollutant models at lag 0 ~ lag 7 days. The distributed lag surface indicated that there was obviously positive association between RD hospitalization and air pollutants (excluding SO2 and O38h), and the adverse effects were observed on the current day for four air pollutants (PM2.5, PM10, NO2, CO). The RRs of higher pollutant concentrations were more fluctuating during the lag period than that by low concentration. The RRs tended to decrease and were sometimes smaller than those at lower concentrations during the lag period. However, no significant association was observed between RD morbidity and SO2 and O38h.
RRs and 95%CIs of air pollutants (per 10 μg/m3 increase and 1 mg/m3 increase in CO) for RD admissions in single-lag models are presented in Table 3. Statistically significant positive associations were shown at lag 02 to lag 07 for PM2.5, at lag 03 to lag 07 for PM10, at lag 0 day in single-day lag and in all cumulative lags for NO2, at lag 01 to lag 07 for CO. And the highest RRs estimates were observed at lag 07 for PM2.5 1.030 (1.012, 1.049), for PM10 1.009 (1.001, 1.015), for NO2 1.047 (1.024, 1.071), and for CO 1.140 (1.052, 1.236), respectively. For particulate matter, the adverse effect of PM2.5 was higher than that of PM10. Obviously, CO had the strongest impact on RD admissions among all pollutants. In Table 3, we can also observe that the RR estimates for gaseous pollutants (e.g., CO, and NO2) were larger than those of particulate matter (e.g., PM2.5 and PM10). Conversely, we found no significantly association between SO2 or O38h and total RD admissions.
Figure 4 illustrates the results of subgroup analysis by different genders in single-pollutant model. When stratified by gender, PM2.5 was statistically significant positive associated with RD hospital admissions for male at lag 02 to lag 07, for female at lag 03 to lag 07, while PM10 showed significant association with RD hospital admissions for male at lag 03 to lag 07, for female at lag 04 to lag 07. NO2 and CO were statistically significant associated with RD hospital admissions for male and female at all cumulative lags. The highest RR estimate was all observed at lag 07 for air pollutants (PM2.5, PM10, NO2, and CO) in male and female, and RR estimate of air pollutants on RD hospital admissions in male was slightly higher than female; it meant that male were more vulnerable to air pollutants exposure except for SO2 and O38h. However, we did not find significant associations between SO2 and O38h and hospitalizations for RD in different genders.
Figure 5 presents the effect estimates of age-specific analyses in different lag days. Different from the whole population, the significant associations of PM2.5, NO2, and CO were observed on lag 0 in children (aged 0–14). In children group (aged 0–14 years old), both PM2.5, PM10, NO2, and CO showed positive associations in all cumulative exposure models (lag01 to lag07), and the most obvious effects were observed on lag07. In aged 15–64 years, only NO2 (lag 01 to lag 04) and CO (lag 02 to lag 05 and lag 07) were significantly associated with hospital admissions for RD. In aged ≥ 65 years, PM2.5 (lag 3, and lag 04 to lag 05), NO2 (lag 01 to lag 07), and CO (lag 02 to lag 04) were significantly associated with hospital admissions for RD. Besides, SO2 (lag 01 to lag 02) was significantly associated with hospital admissions for RD only in children group, and there was no obvious association observed between O38h and hospital admissions for RD in all the three age groups. The strongest associations were observed in aged 0–14 group, and it showed that those 0–14 years were generally more vulnerable to the daily air pollution exposure except for O38h.
Table 4 shows the associations between RD hospitalizations and air pollutants of different lag days by season. The association between air pollutants (PM2.5, PM10, NO2, and CO) and RD morbidity was stronger in cold season (lag2, lag3, and lag 02 to lag 06 for PM2.5, at lag 02 to lag 04 for PM10, at lag 0 to lag 2 day in single-day lag and in all cumulative lags for NO2, at lag2, and lag 01 to lag 07 for CO) than in warm season period. Associations of RD hospitalizations with different exposures (PM2.5, PM10, SO2, NO2, and CO) were positive in warm season period but were not statistically significant. O38h (lag 0 and in all cumulative lags) was negatively associated with RD morbidity in the cold season, but the associations were not significant in the warm season.
Figure 6 shows the exposure–response curves for the associations of six air pollutants (lag07) with RD admission. Overall, all exposure–response curves were almost linear and increasing with no obvious thresholds, except for O38h. For the curve of SO2 and O38h, no association between these two pollutants and RD admission was found.
Table S1 shows the RRs and corresponding 95% CIs of RD at lag07 in the two-pollutant model. Overall, the effects of the two-pollutant models were generally similar to the results in the single-pollutant models. The significance of most pollutants remains robust after the adjustment of co-pollutants. In the sensitivity analyses, the associations of each air pollutant exposure with RD admissions were robust when changing the df for calendar time (6–10 df per year) (Table S2). The results suggest that the model fitted well and results were robust.
Except for SO2 and O38h, significant associations were observed between the levels of four pollutants (PM2.5, PM10, NO2, and CO) and respiratory admissions in the three study hospitals, but the associations of all six air pollutants had no distinct difference across the three hospitals studied (Supplementary Material Fig. S2).
Discussion
In this study, DLNM model was used to analyze the link between air pollutants and hospital admissions for respiratory diseases in Lanzhou from 2014 to 2019, and we observed positive and significant associations between short-term exposure to PM2.5, PM10, NO2, and CO and total RD hospital admissions hospitalization, and the effect estimates were largest on lag 07 days. In the subgroup analysis, we found that exposure to these four pollutants significantly increased the risk of respiratory admission among males and individuals aged 0–14 years. In addition, seasonal variation also impacted the risk of RD patients. The exposure–response curves for the associations between RD and these four pollutants were linear and increasing with no obvious thresholds.
In line with most previous findings, we found that short-term exposure to particulate matter (PM) is positively associated with the daily rates of hospitalizations for RD. For a 10-μg/m3 increase in exposures to PM2.5 and PM10, the effect estimates for RD admissions were 1.030 (95% CI 1.012, 1.049), and 1.009 (95% CI 1.001, 1.015), respectively. Çapraz et al. (2017) found that 10-μg/m3 increase in PM2.5 and PM10 was significantly associated with admissions for RD, with the RR of 1.015 (95% CI 1.011, 1.020) and 1.006 (95% CI 1.003, 1.009), respectively. Similarly, a study in Taiyuan found that a 10-μg/m3 increase in PM2.5 and PM10 concentrations was associated with respiratory admissions, with an RR of 1.005 (95% CI 1.001, 1.010) and 1.004 (95% CI 1.001, 1.007), respectively (Luo et al., 2018). Another study conducted in Hefei, China, also indicated that per 10-μg/m3 increase in PM2.5 at lag 12 days was associated with an RR of 1.068 (1.017 to 1.121) in respiratory morbidity, and each 10-μg/m3 increase in PM10 at lag12 corresponded to an RR of 1.057 (1.010 to 1.107) in respiratory morbidity (Xie et al., 2019). Meanwhile, these studies suggested that PM2.5 had a slightly larger harmful effect than did PM10. Although the relative risks for respiratory diseases in Lanzhou were similar to those findings from previous studies, we found differences in estimates for different cities. The variability of estimates in different cities is influenced by a number of factors, such as demographic and socioeconomic characteristics, PM pollution in various regions, and environmental factors.
SO2 comes mainly from the burning of fossil fuels. With the promotion of clean energy in Lanzhou recently, the concentration of SO2 in the air showed a substantial reducing trend from the beginning of 2014 through to 2019. In this study, the mean concentration of SO2 in Lanzhou was 21.13 μg/m3, which could be considered as low level compared with some megacities and heavily polluted cities including Ho Chi Minh City, Vietnam (30.3 μg/m3) (Phung et al., 2016), Arak, Iran (54.8 μg/m3) (Vahedian et al., 2017), Taiyuan (69.3 μg/m3) (Luo et al., 2018), and Shenyang (55.0 μg/m3) (Chang et al., 2020). Hence, we did not find significant associations between the concentrations of SO2 and RD morbidity. A study in Lanzhou, China (the same area as this study) from 2001 to 2005 showed that the average concentration of SO2 was 79.09 μg/m3 and total RD hospital admissions were significantly increased by 3.4% (RR = 1.034, 95% CI 1.002, 1.067) per inter-quartile range increase in SO2 (Tao et al., 2014). Comparing Tao’s study (Tao et al., 2014) with this study, the mean concentrations of SO2 during different periods showed a decrease, mainly attributable to the large-scale deployment of flue gas desulfurization at China’s power plants (Wang et al., 2014), the strict control of SO2 industrial emissions (Wang et al., 2021), innovation in engine technology and the use of clean fuels. Evidence from Wuhan also showed that cleaner fuel with a lower SO2 levels has been associated with substantial reductions in respiratory morbidity (Wang, 2017). Therefore, improving ambient air quality as a modifiable risk factor has been shown to reduce respiratory diseases.
This study showed positive significant associations between NO2 with respiratory admissions and NO2 had the greater adverse effect on RD hospital admission than PM. Our findings in respiratory morbidity in-line with some previous studies conducted in Asia (Phosri et al., 2019; Phung et al., 2016; Sofwan et al., 2021; Vahedian et al., 2017), Turkey (Çapraz, et al., 2017), and China (Chang et al., 2020; Luo et al., 2018). However, the effect sizes were different among studies, The differences may be associated with NO2 concentration level, various population and their susceptibility, NO2 emission sources distribution, and methods and routes of exposure among different regions and periods, and also may be associated with the different study designs and model specifications.
Until now, studies for the effects of O3 on respiratory admissions are still insufficient and the results remain inconsistent. In this study, we found statistically non-significant association of O3 per10-μg/m3increase with total RD morbidity. Conversely, some other studies from different areas reported protective or adverse effects of O3 on respiratory admissions. On one hand, exposure to low concentrations of O3 might have provided protection from RD morbidity (Chang et al., 2020; Vahedian et al., 2017). On the other hand, estimates for Thailand studies showed elevated risks for respiratory admissions (Phosri et al., 2019). These inconsistent findings indicate the need for further research.
Rarely studies from China have explored the temporal association between CO and hospital admissions for RD. In this time-series study in Lanzhou, CO was found to be positively associated with respiratory admissions and the estimates tended to be stronger at the cumulative lag patterns. An increase of 1 mg/m3 of CO was significantly associated with respiratory admissions, with the highest effect that was observed at lag 07 (RR = 1.140, 95% CI 1.052, 1.236). Phosri et al. (2019) reported that each 1 mg/m3 increase of CO was positively associated with RD morbidity, with an RR of 1.077 (95% CI 1.052, 1.102). Another study conducted in Kuala Lumpur, Malaysia found that ambient CO was associated with an increased risk of RD in single-pollutant model (Sofwan et al., 2021). In China, a study of Shenyang found that the average concentration of CO was 1.02 ± 0.45 mg/m3 (the limit for CO in China is 4 mg/m3), and the study suggested a positive association between CO [RR and 95% CI 1.021 (1.016, 1.025) per 1 mg/m3 increase] and RD morbidity (Chang et al., 2020). In this study, the average concentration of CO was 1.24 ± 0.71 mg/m3 (below the limit for CO in China). Those meant that the effects of CO to RD can be observed at the population level even at low concentrations. The toxicological findings have revealed that exposure to CO may not directly affect respiratory tract, but it is associated with altering the ability of hemoglobin to carry oxygen to peripheral tissues (Manisalidis et al., 2020). Hence, it is possible to exacerbate the symptoms of respiratory diseases.
Subgroup analysis demonstrated that air pollutants were significant associations with respiratory morbidity in various gender and age groups. Through gender-stratified investigation, we discovered that the effects of PM2.5, PM10, NO2, and CO on RD hospital admissions in males were slightly stronger than females, which was similar to results in the study conducted in other foreign cities (Phosri et al., 2019; Vahedian et al., 2017). However, it was inconsistent whether or not gender is a modifier for the association between pollutant and RD patients. For example, some other foreign or Chinese single-city studies in Ho Chi Minh (Phung et al., 2016), Taiyuan (Luo et al., 2018), and Shenyang (Chang et al., 2020) documented that females were more susceptible to air pollution (PM10, SO2, NO2) than males. Such inconsistency could be linked to diverse physiological characteristics in females and males. Additionally, personal characteristics (e.g., routine activity, smoking, occupation, etc.) or socioeconomic status indicate an important factor in air pollution outcomes. The reasons for our gender-specific findings are unclear and need further investigation. In addition, the concentrations of PM2.5, PM10, NO2, and CO were significantly associated with RD morbidity for children (≤ 14 years) and the elderly (≥ 65 years) compared with people aged 15–64 years, which is also supported by previous findings on RD patients (Chang et al., 2020; Fusco et al., 2001; Phosri et al., 2019; Qiu et al., 2018; Vahedian et al., 2017). There are several possible explanations for this increased association with children. First, the child's respiratory system is not fully developed. Second, children have a higher respiratory rate which increases the dose they receive. Third, children generally tended to spend more time outdoors in physical activity than adults (Qiu et al., 2018). For the elderly, they were more vulnerable to exposure to air pollutants, mainly due to pathogen exposure, aging, and comorbidity, which could impair immune defenses and respiratory function, resulting in respiratory infections (Chang et al., 2020; Luo et al., 2018).
In the season-specific analysis, we found that the associations of PM2.5, PM10, NO2, and CO with RD hospitalizations during the cold season were stronger than in the warm season, which were consistent with two previous studies conducted in Shanghai and Shenyang (Chang et al., 2020; Chen et al., 2010). However, other studies have showed different seasonal changes. For example, Qiu et al. (2016) found that the effects estimates of PM on respiratory patients existed a little higher but insignificant effects in warm season than that in cold season. Likewise, Wang et al., (2017) reported that the associations of PM2.5, PM10, NO2, and CO with RD morbidity in cold seasons were stronger than that in hot seasons, but insignificant in two seasons. There are several possible explanations for this phenomenon. Different from the cities or regions studied above, low annual temperature in Lanzhou resulted in burning fossil fuels like coal for heating for up to 5 months a year, where air pollutant levels are high in winter due to wide use of coal heating. As the urban area is located in the valley basin covering 1100 square kilometers, the surrounding mountains make the air hard to circulate. Moreover, less rain and low wind in the cold season may help the accumulation of air pollutants. This phenomenon might be a vital reason for the rise in the amount of patients who were admitted to the hospital for respirator disease in winter. In contrast, we also found that O38h was negatively associated with RD hospital admissions in cold season; these conclusions are inconsistent. In Shenyang, China, respiratory admissions in spring decreased negatively with O3 concentration change (Chang et al., 2020). Wong et al. (1999) study in Hong Kong, China found a positive association between O3 and respiratory diseases admissions in cold season. On the other hand, another study has suggested an adverse effect of ozone on respiratory hospital admissions in warm season (Gu et al., 2020). Altogether, the inconsistency in effects of ozone on respiratory diseases admissions may be dependent on its concentration or people's behavior. For example, O3 concentrations vary significantly in summer and winter; the O3 concentration was significantly higher in summer than in winter. Additionally, in the cold season, people mostly stay indoors, and ventilation is reduced because of heating. Therefore, the effect of O3 exposure should be interpreted with caution, and further investigation is needed to clarify these contradictory results.
The exposure–response relationship curves showed the significantly positive associations between RD hospital admissions and the concentration of PM2.5, PM10, NO2, and CO, and all curves presented similar linear trends without thresholds effect and without peak value, which was almost consistent with some studies. For example, studies in Thailand (PM10, NO2, CO) (Phosri et al., 2019), Shenyang (NO2, CO) (Chang et al., 2020), and Taiyuan (PM2.5, PM10, NO2) (Luo et al., 2018) in China discovered that positive linear relationship between RD hospital admissions and air pollutants. However, a national time-series analysis study on 184 Chinese cities showed that the curves between admissions for RD and PM2.5 or PM10 concentration were nonlinear and had no peak value (Tian et al., 2019). A study in Kuala Lumpur, Malaysia found that all exposure–response curves of PM10, NO2, and CO for respiratory admissions demonstrated a non-linear trend: exposure to air pollutants concentrations demonstrated an increase in the risk of respiratory admissions, then it flattened out (NO2) or decreased (PM10, CO) at higher concentrations (Sofwan et al., 2021). The above differences in the exposure–response curves may be related to air pollutant mixture, the composition of particulate matter, and population sensitivity in different regions and periods.
Some studies have proved the lag in the effect of air pollutants on the RD hospital admissions, and the results varied with regions: in this study, PM2.5, PM10, NO2, and CO at lag 07 days were observed to be the strongest associated with RD hospital admissions. The study in Iran (Vahedian et al., 2017) showed that PM2.5 at lag7, PM10 at lag 0, and CO at lag0 were significantly associated with RD hospitalizations. Study in Thailand (Phosri et al., 2019) showed that PM10, NO2, and CO at lag 01 day were strongest associated with respiratory admission. Study in Turkey (Çapraz et al., 2017) showed that the highest association of each pollutant with total RD hospital admission was observed with PM2.5 and NO2 at lag 4 and PM10 at lag 0. The study in Shenyang, China (Chang et al., 2020) showed that PM10, NO2, and CO at lag0 were observed to be the highest association with daily RD hospital admissions. The study in Taiyuan, China (Luo et al., 2018) showed that PM2.5 at lag 02 days and PM10 and NO2 at lag 05 days were observed to be strongly and significantly associated with RD hospitalization. All those researches are different from the results of this study. The same study area with this paper showed that PM2.5 at lag 07 days was strongest associated with hospital outpatient visits in child with RD (Dong, Wang, et al., 2021b), which was similar to this study. This may be attributed to the special valley terrain of Lanzhou. Besides, Lanzhou City is high in the southeast and low in the northwest, which hinders the horizontal flow of airflow; thus, the lag days were longer in this study. The reasons for the above differences in the studies on the lag effect of air pollution on admission for RD may be related to the topography in different areas, the different levels of air pollution, and different periods (Dong, Wang, et al., 2021b).
The strengths of our study are noteworthy. As we know, this was the first study to examine the association between air pollutants and the risk of respiratory missions in Lanzhou, China. Second, compared with the Poisson regression model, we adopted DLNM to systematically assess the relationships among exposure, lag effect, and RD morbidity. Third, we explored whether the effects of air pollutant on the risk of RD morbidity were affected by season or subgroups (gender and age). Nevertheless, our study has some limitations. First, this study is an ecological study. Although confounding factors such as temperature, humidity, and some gaseous pollutants were adjusted during model fitting, there are still some potential factors (such as use of air conditioners, purifiers use, etc.) that may affect particle toxicity and susceptibility to human health. Furthermore, individual characteristics such as smoking and drinking, marriage and childbirth, occupational history, chronic disease history, medication use, household heating method, cooking fuel usage, indoor stay time daily ventilation time, and other information would affect the morbidity of respiratory. And further researches are needed to confirm our findings. Second, respiratory diseases cover a wide range of specific diseases [such as asthma, collapse of part or all of the lung (pneumothorax or atelectasis), swelling and inflammation in the main passages (bronchial tubes) that carry air to the lungs (bronchitis), chronic obstructive pulmonary disease, lung cancer, lung infection (pneumonia), abnormal buildup of fluid in the lungs (pulmonary edema), even COVID-19)] The associations of overall respiratory diseases with air pollutants do not necessarily imply that each respiratory disease is related to air pollutants. Picking out specific respiratory disease categories in future work will be beneficial. Third, the reasons of respiratory diseases include infection, occupational exposure, smoking, environmental pollution, immune-related respiratory diseases, genetic-related respiratory diseases, and lung manifestations of systemic diseases. Therefore, air pollution is only one of the causes of respiratory diseases, it should be cautious to extrapolate our results. Fourth, the air pollutant concentrations were averaged from at the fixed monitoring sites as a proxy for individual exposure levels, without excluding the influence of spatial heterogeneity of air pollution. It is not completely equivalent to the actual individual exposure concentration, which may reduce the accuracy of the conclusion. Moreover, our research only used the data in Lanzhou, China, and further studies with more samples and multiple cities as well as more related factors should be done for validating the potential spatial heterogeneity of the health effects of air pollutants.
Conclusion
In this study, based on DLNM of time-series analysis, we investigated the effect of air pollutants (PM2.5, PM10, SO2, NO2, O38h, and CO) for respiratory hospital admissions in Lanzhou during 2014–2019. Our results indicate that PM2.5, PM10, NO2, and CO were positively associated with daily hospital admissions for respiratory diseases (RD), and the highest estimated effects were observed at lag 07 days. Among all pollutants, the strongest impact on RD admissions was found in CO, followed by NO2, PM2.5, and PM10. Males and people aged 0–14 group population for RD were more vulnerable to air pollutions, the associations appeared to be more evident in the cold season than in the warm season. The exposure–response curves between RD hospital admissions and the concentration of PM2.5, PM10, NO2, and CO at lag 07 days were almost linear and increasing with no thresholds. Therefore, some effective intervention strategies should be taken to strengthen the treatment of the ambient air pollutants, especially gaseous pollutants (e.g., CO and NO2), thereby, reducing the burden of respiratory disease.
Data availability
Data will be available from the corresponding author on reasonable request.
Abbreviations
- DLNM:
-
Distributed lag nonlinear model
- RR:
-
Relative risk
- CI:
-
Confidence interval
- RD:
-
Respiratory disease
- PM:
-
Particulate matter
- NO2 :
-
Nitrogen dioxide
- SO2 :
-
Sulfur dioxide
- CO:
-
Carbon monoxide
- O38h:
-
Daily 8-h maximum ozone
- SD:
-
Standard deviation
- Min:
-
Minimum
- P25::
-
25Th percentile
- P50::
-
50Th percentile
- P75::
-
75Th percentile
- Max:
-
Maximum
References
Çapraz, Ö., Deniz, A., & Doğan, N. (2017). Effects of air pollution on respiratory hospital admissions in Istanbul, Turkey, 2013 to 2015. Chemosphere, 181, 544–550. https://doi.org/10.1016/j.chemosphere.2017.04.105
Chang, Q., Zhang, H., & Zhao, Y. (2020). Ambient air pollution and daily hospital admissions for respiratory system-related diseases in a heavy polluted city in Northeast China. Environmental Science and Pollution Research International, 27(9), 10055–10064. https://doi.org/10.1007/s11356-020-07678-8
Chen, R., Chu, C., Tan, J., Cao, J., Song, W., Xu, X., Jiang, C., Ma, W., Yang, C., Chen, B., Gui, Y., & Kan, H. (2010). Ambient air pollution and hospital admission in Shanghai, China. Journal of Hazardous Materials, 181(1–3), 234–240. https://doi.org/10.1016/j.jhazmat.2010.05.002
Cheng, B., Ma, Y., Wang, H., Shen, J., Zhang, Y., Guo, L., Guo, Y., & Li, M. (2021). Particulate matter pollution and emergency room visits for respiratory diseases in a valley Basin city of Northwest China. Environmental Geochemistry and Health, 43(9), 3457–3468. https://doi.org/10.1007/s10653-021-00837-x
Cui, J., Halbrook, R. S., Zang, S., Han, S., & Li, X. (2018). Metal concentrations in homing pigeon lung tissue as a biomonitor of atmospheric pollution. Ecotoxicology, 27(2), 169–174. https://doi.org/10.1007/s10646-017-1882-4
Dong, J., Liu, Y., & Bao, H. (2021a). Revalue associations of short-term exposure to air pollution with respiratory hospital admissions in Lanzhou, China after the control and treatment of current pollution. International Journal of Hygiene and Environmental Health, 231, 113658. https://doi.org/10.1016/j.ijheh.2020.113658
Dong, J., Wang, Y., Wang, J., & Bao, H. (2021b). Association between atmospheric PM2.5 and daily outpatient visits for children’s respiratory diseases in Lanzhou. International Journal of Biometeorology, 65(7), 989–999. https://doi.org/10.1007/s00484-021-02080-6
Fang, Z., Huang, C., Zhang, J. J., Xie, J., Dai, S., Ge, E., Xiang, J., Yao, H., Huang, R., Bi, X., Wang, B., Zhong, N., & Lai, K. (2019). Traffic-related air pollution induces non-allergic eosinophilic airway inflammation and cough hypersensitivity in guinea-pigs. Clinical and Experimental Allergy: Journal of the British Society for Allergy and Clinical Immunology, 49(3), 366–377. https://doi.org/10.1111/cea.13308
Fusco, D., Forastiere, F., Michelozzi, P., Spadea, T., Ostro, B., Arcà, M., & Perucci, C. A. (2001). Air pollution and hospital admissions for respiratory conditions in Rome, Italy. European Respiratory Journal, 17(6), 1143–1150. https://doi.org/10.1183/09031936.01.00005501
Gu, J., Shi, Y., & Zhu, Y. (2020). Ambient air pollution and cause-specific risk of hospital admission in China: A nationwide time-series study. PLoS Medicine, 17(8), e1003188. https://doi.org/10.1371/journal.pmed.1003188
Luo, L., Zhang, Y., Jiang, J., Luan, H., Yu, C., Nan, P., Luo, B., & You, M. (2018). Short-term effects of ambient air pollution on hospitalization for respiratory disease in Taiyuan, China: A time-series analysis. International Journal of Environmental Research and Public Health, 15(10), 2160. https://doi.org/10.3390/ijerph15102160
Manisalidis, I., Stavropoulou, E., Stavropoulos, A., & Bezirtzoglou, E. (2020). Environmental and health impacts of air pollution: A review. Frontiers in Public Health, 8, 14. https://doi.org/10.3389/fpubh.2020.00014
Phosri, A., Ueda, K., Phung, V. L. H., Tawatsupa, B., Honda, A., & Takano, H. (2019). Effects of ambient air pollution on daily hospital admissions for respiratory and cardiovascular diseases in Bangkok, Thailand. Science of the Total Environment, 651(Pt 1), 1144–1153. https://doi.org/10.1016/j.scitotenv.2018.09.183
Phung, D., Hien, T. T., Linh, H. N., Luong, L. M., Morawska, L., Chu, C., Binh, N. D., & Thai, P. K. (2016). Air pollution and risk of respiratory and cardiovascular hospitalizations in the most populous city in Vietnam. Science of the Total Environment, 557–558, 322–330. https://doi.org/10.1016/j.scitotenv.2016.03.070
Qiu, H., Sun, S., Tang, R., Chan, K. P., & Tian, L. (2016). Pneumonia hospitalization risk in the elderly attributable to cold and hot temperatures in Hong Kong, China. American Journal of Epidemiology, 184(8), 555–569. https://doi.org/10.1093/aje/kww041
Qiu, H., Yu, H., Wang, L., Zhu, X., Chen, M., Zhou, L., Deng, R., Zhang, Y., Pu, X., & Pan, J. (2018). The burden of overall and cause-specific respiratory morbidity due to ambient air pollution in Sichuan Basin, China: A multi-city time-series analysis. Environmental Research, 167, 428–436. https://doi.org/10.1016/j.envres.2018.08.011
Sofwan, N. M., Mahiyuddin, W. R. W., Latif, M. T., Ayub, N. A., Yatim, A. N. M., Mohtar, A. A. A., Othman, M., Aizuddin, A. N., & Sahani, M. (2021). Risks of exposure to ambient air pollutants on the admission of respiratory and cardiovascular diseases in Kuala Lumpur. Sustainable Cities and Society, 75, 103390. https://doi.org/10.1016/j.scs.2021.103390
Tao, Y., Mi, S., Zhou, S., Wang, S., & Xie, X. (2014). Air pollution and hospital admissions for respiratory diseases in Lanzhou, China. Environmental Pollution, 185, 196–201. https://doi.org/10.1016/j.envpol.2013.10.035
Tian, Y., Liu, H., Wu, Y., Si, Y., Li, M., Wu, Y., Wang, X., Wang, M., Chen, L., Wei, C., Wu, T., Gao, P., & Hu, Y. (2019). Ambient particulate matter pollution and adult hospital admissions for pneumonia in urban China: A national time series analysis for 2014 through 2017. PLoS Medicine, 16(12), e1003010. https://doi.org/10.1371/journal.pmed.1003010
Vahedian, M., Khanjani, N., Mirzaee, M., & Koolivand, A. (2017). Associations of short-term exposure to air pollution with respiratory hospital admissions in Arak, Iran. Journal of Environmental Health Science & Engineering, 15(1), 17. https://doi.org/10.1186/s40201-017-0277-z
Wang, S. X., Zhao, B., Cai, S. Y., Klimont, Z., Nielsen, C. P., Morikawa, T., Woo, J. H., Kim, Y., Fu, X., Xu, J. Y., Hao, J. M., & He, K. B. (2014). Emission trends and mitigation options for air pollutants in East Asia. Atmospheric Chemistry and Physics, 14(13), 6571–6603. https://doi.org/10.5194/acp-14-6571-2014
Wang, X. Y. (2017). The effects of air pollution on daily respiratory and cardiovascular diseases hospital admissions in Wuhan. Thesis. Huazhong University of Science and Technology. https://doi.org/10.7666/d.D01308067
Wang, Z., Shi, X., Pan, C., & Wang, S. (2021). Spatial and temporal characteristics of environmental air quality and its relationship with seasonal climatic conditions in eastern China during 2015–2018. International Journal of Environmental Research and Public Health, 18(9), 4524. https://doi.org/10.3390/ijerph18094524
Wong, T. W., Lau, T. S., Yu, T. S., Neller, A., Wong, S. L., Tam, W., & Pang, S. W. (1999). Air pollution and hospital admissions for respiratory and cardiovascular diseases in Hong Kong. Occupational and Environmental Medicine, 56(10), 679–683. https://doi.org/10.1136/oem.56.10.679
Xie, J., Teng, J., Fan, Y., Xie, R., & Shen, A. (2019). The short-term effects of air pollutants on hospitalizations for respiratory disease in Hefei, China. International Journal of Biometeorology, 63(3), 315–326. https://doi.org/10.1007/s00484-018-01665-y
Xu, M., Hu, P., Chen, R., Liu, B., Chen, H., Hou, J., Ke, L., Huang, J., Ren, H., & Hu, H. (2021). Association of long-term exposure to ambient air pollution with the number of tuberculosis cases notified: A time-series study in Hong Kong. Environmental Science and Pollution Research. https://doi.org/10.1007/s11356-021-17082-5
Acknowledgements
The daily meteorological data near the ground of Lanzhou were obtained from China meteorological data service centre (http://data.cma.cn/). We also acknowledge all anonymous reviewers for their insightful and valuable comments.
Funding
Financial support for this study was provided by the National Science Foundation of China (No. 41975019), Scientific Research and Innovation Foundation of Gansu University of Chinese Medicine (No. 2020KCYB-4), and the Fundamental Research Funds for the Central University (lzujbky-2019-42).
Author information
Authors and Affiliations
Contributions
LJ: Conceptualization; data collection, data compilation; data analysis; Methodology; writing original draft and graphics. TZ: Conceptualization; data collection; data analysis; and the manuscript revises. SF and XZ: Collating and checking data, and graphics. YB: Conceptualization and data collection. All gave final approval and agree to be accountable for all aspects of work ensuring integrity and accuracy.
Corresponding author
Ethics declarations
Competing interest
The authors declare that they have no competing interests.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jin, L., Zhou, T., Fang, S. et al. Association of air pollutants and hospital admissions for respiratory diseases in Lanzhou, China, 2014–2019. Environ Geochem Health 45, 941–959 (2023). https://doi.org/10.1007/s10653-022-01256-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-022-01256-2