Abstract
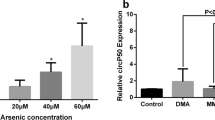
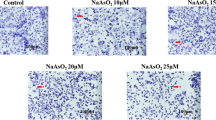
Arsenic is a wildly distributed carcinogen in the environment. Arsenic-induced apoptosis has been extensively studied in therapeutics and toxicology. LncRNA MEG3 has been extensively studied as apoptosis regulatory gene in recent years. However, it stays unclear regarding how the mechanism of MEG3 regulates arsenic-induced apoptosis. Our focus was to explore the effects of MEG3 on arsenic-induced apoptosis. MTS assay was used to test cell viability, and qRT-PCR was for the examination of gene expressions. The effect of the apoptosis and necrosis after knockdown MEG3 was detected with double staining. Our results demonstrated that MEG3 expression was positively correlated with the concentration of three arsenic species (inorganic arsenic (iAs), monomethylarsonic acid (MMA) and dimethylarsinic acid (DMA)) (p < 0.05). The ability of iAs to induce MEG3 expression was much higher compared with that induced by MMA and DMA. In addition, our experiments confirmed that MEG3 knockdown increased cell viability and arsenic-induced apoptosis, but cell viability decreased after iAs treatment. Moreover, LncRNA MEG3 regulated apoptosis via down-regulate API5 while up-regulate CASP7, CCND3 and APAF1. It is further proved that arsenic-induced apoptosis increased after the knockdown of MEG3, which regulates these genes. These findings provide experimental evidence and possible mechanisms for subsequent research on the effects of arsenic on health.






Similar content being viewed by others
References
Ashley-Martin, J., Dodds, L., Arbuckle, T. E., Bouchard, M. F., Shapiro, G. D., Fisher, M., et al. (2018). Association between maternal urinary speciated arsenic concentrations and gestational diabetes in a cohort of Canadian women. Environment International, 121(Pt 1), 714–720.
Basset, C., Bonnet-Magnaval, F., Navarro, M. G., Touriol, C., Courtade, M., Prats, H., et al. (2017). Api5 a new cofactor of estrogen receptor alpha involved in breast cancer outcome. Oncotarget, 8(32), 52511–52526.
Bozack, A. K., Saxena, R., & Gamble, M. V. (2018). Nutritional influences on one-carbon metabolism: Effects on arsenic methylation and toxicity. Annual Review of Nutrition, 38, 401–429.
Cheng, B., Yang, X., An, L., Gao, B., & Liu, X. (2010). Arsenic trioxide-induced apoptosis of Hep-2 cell line through modulating intracellular glutathione (GSH) level. Auris, Nasus, Larynx, 37(1), 89–94.
Cheng, H., Qin, M., Chen, Y., Dong, H., Shao, Y., Ren, S., et al. (2018). Expression changes of tumor-related gene mrna in workers who smelt arsenic. Journal of Hygiene Research, 47(4), 577–587.
Deng, D., & Liang, H. (2020). Silencing meg3 protects PC12 cells from hypoxic injury by targeting miR-21. Artificial Cells, Nanomedicine, and Biotechnology, 48(1), 610–619.
Dodmane, P. R., Arnold, L. L., Kakiuchi-Kiyota, S., Qiu, F., Liu, X., Rennard, S. I., et al. (2013). Cytotoxicity and gene expression changes induced by inorganic and organic trivalent arsenicals in human cells. Toxicology, 312, 18–29.
Dong, X., Wang, J., Li, T., Xu, Y. P., & Li, S. Y. (2018). Down regulation of lncRNA meg3 promotes colorectal adenocarcinoma cell proliferation and inhibits the apoptosis by up-regulating TGF-β1 and its downstream sphingosine kinase 1. European Review for Medical and Pharmacological Sciences, 22(23), 8265–8272.
Du, J., Zhou, N., Liu, H., Jiang, F., Wang, Y., Hu, C., et al. (2012). Arsenic induces functional re-expression of estrogen receptor α by demethylation of DNA in estrogen receptor-negative human breast cancer. PLoS ONE, 7(4), e35957.
Fan, Z., He, J., Fu, T., Zhang, W., Yang, G., Qu, X., et al. (2019). Arsenic trioxide inhibits EMT in hepatocellular carcinoma by promoting lncRNA meg3 via PKM2. Biochemical and Biophysical Research Communications, 513(4), 834–840.
Ghafouri-Fard, S., & Taheri, M. (2019). Maternally expressed gene 3 (meg3): A tumor suppressor long non coding RNA. Biomedicine & Pharmacotherapy, 118, 109129.
Gholizadeh, M. A., Shamsabadi, F. T., Yamchi, A., Golalipour, M., Jhingan, G. D., & Shahbazi, M. (2020). Identification of hub genes associated with RNAi-induced silencing of XIAP through targeted proteomics approach in MCF7 cells. Cell & Bioscience, 10, 78.
Gong, L., Xu, H., Chang, H., Tong, Y., Zhang, T., & Guo, G. (2018). Knockdown of long non-coding RNA MEG3 protects H9c2 cells from hypoxia-induced injury by targeting microRNA-183. Journal of Cellular Biochemistry, 119(2), 1429–1440.
Gribble, M. O., Voruganti, V. S., Cole, S. A., Haack, K., Balakrishnan, P., Laston, S. L., et al. (2015). Linkage analysis of urine arsenic species patterns in the strong heart family study. Toxicological Sciences : An Official Journal of the Society of Toxicology, 148(1), 89–100.
Hayakawa, T., Kobayashi, Y., Cui, X., & Hirano, S. (2005). A new metabolic pathway of arsenite: Arsenic-glutathione complexes are substrates for human arsenic methyltransferase Cyt19. Archives of Toxicology, 79(4), 183–191.
He, Y., Zhang, R., Song, X., Shang, L., Wu, X., & Huang, D. (2018). Inorganic arsenic exposure increased expression of fas and bax gene in vivo and vitro. Gene, 671, 135–141.
He, Y., Zhang, R., Chen, J., Tan, J., Wang, M., & Wu, X. (2020). The ability of arsenic metabolism affected the expression of lncRNA PANDAR, DNA damage, or DNA methylation in peripheral blood lymphocytes of laborers. Human & Experimental Toxicology, 39(5), 605–613.
Jiang, X., Chen, C., Gu, S., & Zhang, Z. (2018). Regulation of ABCG2 by nuclear factor kappa B affects the sensitivity of human lung adenocarcinoma A549 cells to arsenic trioxide. Environmental Toxicology and Pharmacology, 57, 141–150.
Khairul, I., Wang, Q. Q., Jiang, Y. H., Wang, C., & Naranmandura, H. (2017). Metabolism, toxicity and anticancer activities of arsenic compounds. Oncotarget, 8(14), 23905–23926.
Kinoshita, A., Wanibuchi, H., Wei, M., Yunoki, T., & Fukushima, S. (2007). Elevation of 8-hydroxydeoxyguanosine and cell proliferation via generation of oxidative stress by organic arsenicals contributes to their carcinogenicity in the rat liver and bladder. Toxicology and Applied Pharmacology, 221(3), 295–305.
Kuo, C. C., Moon, K. A., Wang, S. L., Silbergeld, E., & Navas-Acien, A. (2017). The Association of arsenic metabolism with cancer, cardiovascular disease, and diabetes: A systematic review of the epidemiological evidence. Environmental Health Perspectives, 125(8), 087001.
Liu, F., Chen, Y., Liu, R., Chen, B., Liu, C., & Xing, J. (2020). Long noncoding RNA (meg3) in urinal exosomes functions as a biomarker for the diagnosis of hunner-type interstitial cystitis (HIC). Journal of Cellular Biochemistry, 121(2), 1227–1237.
Luo, D., Zhang, X., Du, R., Gao, W., Luo, N., Zhao, S., et al. (2018). Low dosage of arsenic trioxide (As2O3) inhibits angiogenesis in epithelial ovarian cancer without cell apoptosis. Journal of Biological Inorganic Chemistry, 23(6), 939–947.
Ma, L., Wang, F., Du, C., Zhang, Z., Guo, H., Xie, X., et al. (2018). Long non-coding RNA meg3 functions as a tumour suppressor and has prognostic predictive value in human pancreatic cancer. Oncology Reports, 39(3), 1132–1140.
Mochizuki, H. (2019). Arsenic Neurotoxicity in Humans. International Journal of Molecular Sciences, 20(14), 3418.
Naranmandura, H., Suzuki, N., & Suzuki, K. T. (2006). Trivalent arsenicals are bound to proteins during reductive methylation. Chemical Research in Toxicology, 19(8), 1010–1018.
Rasheed, H., Kay, P., Slack, R., & Gong, Y. Y. (2018). The effect of association between inefficient arsenic methylation capacity and demographic characteristics on the risk of skin lesions. Toxicology and Applied Pharmacology, 339, 42–51.
Sinha, D., & Prasad, P. (2020). Health effects inflicted by chronic low-level arsenic contamination in groundwater: A global public health challenge. Journal of Applied Toxicology, 40(1), 87–131.
Smith, A. H., Marshall, G., Roh, T., Ferreccio, C., Liaw, J., & Steinmaus, C. (2018). Lung, bladder, and kidney cancer mortality 40 years after arsenic exposure reduction. Journal of the National Cancer Institute, 110(3), 241–249.
Wang, H., Cheng, J., Wang, H., Wang, M., Zhao, J., & Wu, Z. (2019a). Protective effect of apple phlorizin on hydrogen peroxide-induced cell damage in HepG2 cells. Journal of food Biochemistry, 43(12), e13052.
Wang, W., Xie, Y., & Chen, F. (2019b). LncRNA meg3 acts a biomarker and regulates cell functions by targeting ADAR1 in colorectal cancer. World Journal of Gastroenterology, 25(29), 3972–3984.
Wen, W., Lu, L., He, Y., Cheng, H., He, F., Cao, S., et al. (2016). LincRNAs and base modifications of p53 induced by arsenic methylation in workers. Chemico-biological Interactions, 246, 1–10.
Wu, J. L., Meng, F. M., & Li, H. J. (2018). High expression of lncRNA meg3 participates in non-small cell lung cancer by regulating microRNA-7-5p. European Review for Medical and Pharmacological Sciences, 22(18), 5938–5945.
Yang, Z., Bian, E., Xu, Y., Ji, X., Tang, F., Ma, C., et al. (2020). Mega3 induces EMT and invasion of glioma cells via autophagy. Onco Targets and Theraphy, 13, 989–1000.
Yin, L., & Yu, X. (2018). Arsenic-induced apoptosis in the p53-proficient and p53-deficient cells through differential modulation of NFkB pathway. Food and Chemical Toxicology: An International Journal Published for British Industrial Biological Research Association, 118, 849–860.
Zhang, Q., Li, Y., Liu, J., Wang, D., Zheng, Q., & Sun, G. (2014). Differences of urinary arsenic metabolites and methylation capacity between individuals with and without skin lesions in Inner Mongolia, Northern China. International Journal of Environmental Research and Public Health, 11(7), 7319–7332.
Zhang, W., Cui, X., Gao, Y., Sun, L., Wang, J., Yang, Y., et al. (2019a). Role of pigment epithelium-derived factor (PEDF) on arsenic-induced neuronal apoptosis. Chemosphere, 215, 925–931.
Zhang, X., Wu, N., Wang, J., & Li, Z. (2019b). LncRNA meg3 inhibits cell proliferation and induces apoptosis in laryngeal cancer via miR-23a/APAF-1 axis. Journal of Cellular and Molecular Medicine, 23(10), 6708–6719.
Zhang, Y., Li, Y., Luo, L., He, Q., Gao, Y., Feng, H., et al. (2019c). Factors affecting differential methylation of dna promoters in arsenic-exposed populations. Biological Trace Element Research, 189(2), 437–446.
Zhou, J., Li, G., Han, G., Feng, S., Liu, Y., Chen, J., et al. (2020). Emodin induced necroptosis in the glioma cell line U251 via the TNF-α/RIP1/RIP3 pathway. Investigational New Drugs, 38(1), 50–59.
Zhu, M., Wang, X., Gu, Y., Wang, F., Li, L., & Qiu, X. (2019). Meg3 overexpression inhibits the tumorigenesis of breast cancer by downregulating miR-21 through the PI3K/Akt pathway. Archives of Biochemistry and Biophysics, 661, 22–30.
Zou, D. M., Zhou, S. M., Li, L. H., Zhou, J. L., Tang, Z. M., & Wang, S. H. (2020). Knockdown of long noncoding rnas of maternally expressed 3 alleviates hyperoxia-induced lung injury via inhibiting thioredoxin-interacting protein-mediated pyroptosis by binding to mir-18a. The American Journal of Pathology, 190(5), 994–1005.
Acknowledgements
This work was financially sponsored by the National Natural Science Foundation of China (Grant No. 81860572).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
No potential conflicts of interest were disclosed.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, M., Tan, J., Jiang, C. et al. Inorganic arsenic influences cell apoptosis by regulating the expression of MEG3 gene. Environ Geochem Health 43, 475–484 (2021). https://doi.org/10.1007/s10653-020-00740-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-020-00740-x