Abstract
Climate change significantly impacts marine ecosystems worldwide, leading to alterations in the composition and structure of marine communities. In this study, we aim to explore the effects of temperature on demersal fish communities in the Central Mediterranean Sea, using data collected from a standardized monitoring program over 23 years. Computationally efficient Bayesian inference is performed using the integrated nested Laplace approximation and the stochastic partial differential equation approach to model the spatial and temporal dynamics of the fish communities. We focused on the mean temperature of the catch (MTC) as an indicator of the response of fish communities to changes in temperature. Our results showed that MTC decreased significantly with increasing depth, indicating that deeper fish communities may be composed of colder affinity species, more vulnerable to future warming. We also found that MTC had a step-wise rather than linear increase with increasing water temperature, suggesting that fish communities may be able to adapt to gradual changes in temperature up to a certain threshold before undergoing abrupt changes. Our findings highlight the importance of considering the non-linear dynamics of fish communities when assessing the impacts of temperature on marine ecosystems and provide important insights into the potential impacts of climate change on demersal fish communities in the Central Mediterranean Sea.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Human activities are deeply impacting the global climate with strong consequences to the biotic and abiotic components of the ocean ecosystems (Hoegh-Guldberg and Bruno 2010). Climatic phenomena and global warming are recognized to be the main drivers for sea temperature increase (Levitus et al. 2000). These drives may lead to changes in the biological/physiological characteristics of fish populations, including somatic growth, maximum consumption rate and metabolic rate (Gillooly et al. 2001; Brown et al. 2004). Climate change impacts also on species phenology and related reproductive aspects (e.g. onset and duration of spawning (Poloczanska et al. 2013)) and, as they are strongly dependent on the physiological aspects, species may respond to ocean warming by modifying their vertical distribution (Dulvy et al. 2008) and/or latitudinal range (Perry et al. 2005).
Over the years, the changes due to ocean warming led to a profound and documented impact on fisheries. Specifically, these changes impact on increasing the warm-water species in fish communities and, in turn, in fisheries catch (Cheung et al. 2010; Cheung et al. 2013; Baptista et al. 2014). Therefore, assessing the effects of climate change on fisheries is one of the challenges for their sustainable management (Leitão et al. 2014). The mean temperature of the catch (MTC) is a well-recognized index to assess the effects of climate change, especially in terms of warming, on fisheries catches (Cheung et al. 2013; Valente et al. 2023). Particularly, the MTC, computed from the average inferred temperature preference of exploited species and weighted by their biomass in the catch, correlates with a trend in sea surface temperature (Cheung et al. 2013). MTC was recently used to assess the effect of ocean warming both in large marine ecosystems and at local level: e.g. the Mediterranean (Tsikliras and Stergiou 2014; Tsikliras et al. 2015; Keskin and Pauly 2014; Valente et al. 2023), Caribbean (Maharaj et al. 2018) and Adriatic Sea (Fortibuoni et al. 2015).
The Mediterranean Sea has a long history of fishing activity that has led to the overexploitation of most of the Mediterranean fish stocks (Colloca et al. 2013), increasing their vulnerability to climate variability. In fact, Sea Surface Temperature (SST) is increasing at a higher rate than the global average, leading to fast changes in the catch composition of fisheries but with controversial results. Particularly, in the eastern part of the Mediterranean Sea, the temperature increase has a most evident impact on the marine faunal composition, which has also been altered by alien species from the Suez Canal (Tsikliras and Stergiou 2014). On the contrary, Fortibuoni et al. (2015) did not found any significant change in MTC of fisheries catches of the Adriatic Sea. Understanding how climatic variability is affecting MTC is also relevant for predicting future changes in fish communities and related fisheries catches under different climatic scenarios. Moreover, biological systems’ responses to environmental changes can be shifted over time. (Legendre and Legendre 1998; Olden and Neff 2001). The scale of this delay is variable and is related to the frequency of occurrence of the event (e.g. daily, monthly or inter-annually) in relation to the life cycle of the different taxonomic groups being examined. For instance, variations in phytoplankton species response can be observed in relatively short time periods (e.g. hours or days) (Li et al. 2009; Chen et al. 2010; Vidal et al. 2010), whereas fishes usually respond over longer time scales (e.g. months or years) (Parraga et al. 2010; Qiu et al. 2010; Von Biela et al. 2011). In the case of demersal communities exploited by fishing, understanding the temporal relationship between environmental changes and community variations could be helpful for adequate resource management.
In this study, we apply a Bayesian spatio-temporal statistical framework to assess the change in the MTC index using trawl surveys’ biomass indices in a Mediterranean fish biodiversity hotspot (Di Lorenzo et al. 2018). We estimate a spatio-temporal model using the Integrated Nested Laplace Approximation (INLA) and the Stochastic Partial Differential Equation (SPDE) approaches with different potential predictors to disentangle the role of different driving forces on MTC changes. We use this approach to efficiently take into account the effect of spatial correlation and describe the temporal evolution of MTC. Moreover, we compare different time lags between environmental temperature and MTC level in order to highlight how long a change in environmental temperature takes to express its effect on faunal composition. To the best of our knowledge, this is the first time that this methodological approach has been applied to investigate the relationship between climate change and the thermal affinity of marine communities.
The paper is structured as follows: in Sect. 2, a description of the study area and the motivating data is given. In Sect. 3, the statistical methods for spatial data used in this research are described. Section 4 is devoted to a discussion of results, and in Sect. 5 some brief conclusions are reported. Supplementary material is provided in the Appendix (Section A).
2 Materials
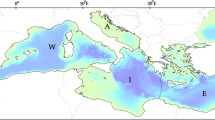
The Strait of Sicily is a transition area in the south-central Mediterranean Sea connecting the Western and Eastern Mediterranean sectors. Along the southern coast of Sicily (south Italy), the continental shelf is characterized by two wide and shallow (100 m depth) banks in the western (Adventure Bank) and eastern sectors (Malta Bank), separated by a narrow shelf in the middle part. Recent studies highlighted that this area is a biodiversity hotspot in the Mediterranean Sea, including a high diversity and biomass of demersal communities over the offshore detritic bottoms of the Adventure Bank (Consoli et al. 2016; Di Lorenzo et al. 2018).
We collected georeferenced biomass indices of fish within the demersal trawl surveys MEDITS (Mediterranean International Trawl Survey program (Bertrand et al. 2002)), performed in the study area between 1995 and 2018. The MEDITS survey is carried out annually in late spring-early summer, providing a long-term dataset of fishery-independent data relating to demersal species abundance, demographic structure, and spatial distribution. Sampling followed a random design stratified by depth (depth strata: 10–50 m, 51–100 m, 101–200 m, 201–500 m, 501–800 m) with the number of haul per stratum proportional to each stratum surface.
In the present work, only the hauls located on the continental shelf (depth \(<200\) m) are considered, assuming that the organisms that inhabit this area have a greater probability of being influenced by changes in the sea temperature (Fig. 1).
At each trawl station, fish species are sorted, weighted, counted and measured, and their relative abundance is expressed as \(\mathrm {kg/km}^2\). Each species’ preferred temperature (median, 25th and 75th percentile) is acquired from the online database FishBase (http://www.fishbase.org). The MTC was then calculated for each haul as the average of the temperature preference of all the exploited fish species weighted by their annual MEDITS catch, that is
where \(C_{hti}\) are the catches of species i for year t in haul h, \(T_i\) is the median temperature preference of species i and n is the total number of species in the annual catch. An increase in the level of MTC in an area indicates a change in the thermophilic composition of the MEDITS catch, which suggests an increase in the dominance of warm-water species in that area (Cheung et al. 2013).
In order to assess the temporal and spatial changes of the MTC in the Strait of Sicily, several factors, which are assumed to be related to the MTC level, were also considered: the annual mean of the SST and the annual mean of the Bottom Sea Temperature (BST, both measured in Celsius degrees); the depth of the catch, categorized in three levels (low: [10–60 m], medium: (60–100 m], high: [100–200 m], based on a hierarchical cluster performed on community Bray-Curtis dissimilarities matrix); and the spatial (latitude and longitude) and temporal (year) coordinates.
Raster annual maps of SST and BST were constructed by averaging monthly continuous digital maps (downloaded from the website http://marine.copernicus.eu—MEDSEA MULTIYEAR PHY 006 004–0.042° × 0.042° pixel resolution). Also, considering that a change in temperature may take time to express its effects on the faunal composition, time lags of one, two and three years were considered in modelling the effects of SST and BST on MTC. Initially, variables related to undersea currents and salinity (downloaded from the website http://marine.copernicus.eu - MEDSEA MULTIYEAR PHY 006 004–0.042° × 0.042° pixel resolution) were also taken into account by means of a preliminary analysis, from which, however, there was no evidence of any significant impact on the level of the MTC; so, they were excluded from the study.
Figure 2 shows the smoothed trend of BST during the time interval 1995–2018, according to each level of depth.
The BST trend appears to have almost constantly increased in shallow areas, which are likely to be the most susceptible to changes in external environmental temperature.
3 Methods
3.1 General framework
In general, spatial data can be considered as realizations of a stochastic process indexed in space (Cressie 1993):
where \(\varvec{s}\) is the vector of spatial coordinates associated to Y in a d-dimensional euclidean space and D is the observed portion of space, with \(D\in {\mathbb {R}}^d\). In the case of \(d=2\), generally \(\varvec{s}\) contains latitude and longitude of Y. In particular, in the geostatistical approach (see, e.g. Cressie and Wickle (2011)) point-referenced data consists of a set of measurements (realizations) of Y taken at a finite set of points \(\{\varvec{s}_1,\dots ,\varvec{s}_n\}\) in D, and the spatial index \(\varvec{s}\) can take any value in the continuum in D. The interest, typically, is in inferring the main characteristics of the spatial process such as its mean and variability and predicting values of Y at unobserved points in space using information derived from the analysis of the observed data \(\varvec{y}\).
An useful approach for the estimation of a geostatistical model is to assume that there is a spatially continuous variable underlying the observations that can be modeled using a Gaussian random field (GRF) (Abrahamsen 1997) \(U(\varvec{s})\), which is a random function for which holds that, for every finite set of points \(\{\varvec{s}_1, \dots , \varvec{s}_n\}\),
where \(\varvec{u} = \{u(\varvec{s}_1), \ldots , u(\varvec{s}_n)\}\) is a realization of \(U(\varvec{s})\) at \(n\) locations, and \(\varvec{\mu }\) and \(\varvec{\Sigma }\) are the mean vector and the covariance matrix of the process, respectively.
The GRF incorporates the correlation structure of the process by means of its covariance matrix \(\varvec{\Sigma } = (\Sigma _{i,j})\), \(i, j = (1, \dots , n)\), which is constructed from a covariance function. A common choice for the specification of the covariance function, which is of main interest here, is the Matérn function (Matérn 1960), which implies that each single element \(\Sigma _{ij}\) of the covariance matrix \(\varvec{\Sigma }\) is defined as
where \(\sigma _u^2\) is the marginal variance of the process, \(\nu >0\) is the smoothing parameter, \(k>0\) is a scale parameter, \(||\varvec{s}_i - \varvec{s}_j||\) is the euclidean distance between \(\varvec{s}_i\) and \(\varvec{s}_j\) and \(K_{\nu }\) is the modified Bessel function of second kind and order \(\nu >0\). Instead of the parameter k, for better interpretability we generally consider the range parameter, i.e., the distance such that the spatial correlation between two points is very small; in our case the range (about 0.14) has been defined empirically by the following relationship (Lindgren and Rue 2015):
Although the use of GRFs proves convenient due to their good analytical properties, parameter estimation is often problematic in practice, especially with large data sets. Indeed, inference on the parameters of equation (4) has a computational cost equal to \({\mathcal {O}}(n^3)\), as it requires factoring fully dense \(n \times n\) covariance matrices (Lindgren et al. 2011). Furthermore, the fitting of a model in a Bayesian inferential paradigm is traditionally based on Markov chain Monte Carlo algorithms, which require these calculations at each iteration, making the task even more difficult. In this regard, see Banerjee et al (2003, p. 387).
An approach to overcome this problem, which is based on stochastic partial differential equations (SPDE), has been developed by Lindgren et al. (2011). The method consists in approximating a (continuous) Matérn GRF with a spatial process with a discrete index (i.e. a Gaussian Markov Random Field (GMRF)). This discretization of the study region, which is called a mesh, consists of a basis function representation defined on a triangulation of the spatial domain D; the vertices of the triangles are called nodes, and each node corresponds to a basis function. Therefore, due to the sparsity of the precision matrix of GMRFs (which is induced by the conditional independence structure of the process), it is possible to use appropriate computational techniques for sparse matrices (for an extensive description, see Rue and Held (2005)). For a GMRF model in \({\mathbb {R}}^2\), the computational cost is typically \(O(n^{3/2})\) (Lindgren et al. 2011), which is a significant improvement over the \(O(n^3)\) cost of the Gaussian field (GF). The use of such computational techniques allows the model to be estimated efficiently, within the Bayesian framework, using INLA (Rue et al. 2009).
3.2 The spatio-temporal model for MTC
Let \(y(\varvec{s},t)\) denote the observed value of the MTC level measured at location \(\varvec{s}\) and year \(t = 1, \dots , T\). We assume that
where \(\beta _0\) is a regression intercept, \(\epsilon ({\varvec{s},t}) \sim {\mathcal {N}}(0, \sigma ^2_{\epsilon })\) is the measurement error, \(\varvec{x}({\varvec{s},t})\) is a vector of covariates (namely, BST and Depth) with corresponding vector of regression coefficients \(\varvec{\beta }\), and \(u\mid (\sigma _u^2,r)\) is a realization of the Gaussian spatial field at location \(\varvec{s}\), which is represented by its GMRF approximation according to the SPDE approach. Online Fig. A1 shows the measurement locations (red dots) within the mesh in two dimensions; details about how we set the parameters related to the mesh construction are reported in the caption (general information about the mesh construction criteria can be found in Krainski et al. (2019)).
Moreover, in order to investigate the overall trend of the MTC according to each level l of depth, a random walk \({v_{l}(t)}\) of order 1 has been included into the model as a smoothing latent component. A detailed description of the use of random walk models for smoothing methods with INLA can be found in Wang et al. (2018) to take care of possible non-linear relationship, with
where \(\sigma ^2_v\), together with \(\sigma ^2_{\epsilon}\), controls the smoothness of the function.
Because no prior information was available, a non-informative zero-mean Gaussian prior distribution was used for the parameters \(\beta _0\) and \(\varvec{\beta }\). Also, the GMRF prior (according to the SPDE approach) and the random walk prior in Equation (7) were assigned to \(\varvec{u}\) and \(\varvec{v}\), respectively. Hence, the latent field \(\varvec{\theta }=(\beta _0,\varvec{\beta },\varvec{u}, \varvec{v})\) is jointly Gaussian with hyperparameter vector \(\varvec{\psi _1}=(\sigma _u, r, \sigma _v)\). The observations y are assumed to be independently Normally distributed given \(\varvec{\theta }\) and \(\varvec{\psi _2}=\sigma _{\epsilon }\).
Denoting the vector of all hyperparameters by \(\varvec{\psi }=(\varvec{\psi _1}, \varvec{\psi _2})\), the joint posterior distribution is then
The posterior marginal distributions for each component of \(\varvec{\theta }\) and \(\varvec{\psi }\) are estimated using INLA (for further details see, e.g., Rue et al. (2009) and Rue et al. (2017)).
4 Results and discussion
The posterior densities of regression coefficients and hyperparameters, estimated by the INLA-SPDE model, are shown in Online Figs. A2 and A3, respectively. Their relevant statistics are reported in Table 1, along with their 95% HPD (Highest Posterior Density) intervals. Graphical representations of model results and checks can also be found in Section A (Online Figs. A4 and A5).
The estimated posterior mode of the standard deviation (SD) of the SPDE random component (0.57 °C) suggests that its inclusion into the model allowed to capture some spatial heterogeneity unexplained by the other covariates. This can also be seen by looking at Online Fig. A4, which shows the estimated posterior mean of the SPDE component across the study region. Online Fig. A5 shows the estimated Matérn correlation as a function of distance. Considering the dimension of the rectangle which contains the study area (about 374.6\(\times\)130.8 km), spatial correlation seems to decrease fairly quickly (the posterior mode of the range is \(19^\circ \approx 21.06 \, \text {km}\)), suggesting a relatively high variability in the spatial distribution of species. Online Fig. A8 illustrates the estimated effect of the Random Walk smoothing component, which allowed to capture the non-linear temporal evolution of the MTC. This effect also appears to vary across different depth strata.
Using BST and not SST as the environmental temperature variable, led to a decrease in Deviance Information Criterion (Spiegelhalter et al. 2002) (DIC) equal to 6.39. BST turned out to have a significant effect on the MTC level, with the best fit given by its three years lag (DIC values are 2444, 2450, 2443 and 2442 for lag 0, 1, 2, 3, respectively). We also run a 10-fold cross-validation (Hastie et al. 2009) comparing SST and the different lags of BST. We considered the Root Mean Square Error (RMSE) as the out-of-sample performance measure, computed using the differences between posterior means of the MTC and the observed values (RMSE results are 1.2154, 1.2104, 1.2193, 1.2155 and 1.2103 for SST and BST lag 0, 1, 2, 3, respectively). Although the comparison based on the results of DIC and cross-validation showed rather marginal differences, a preference emerged for the inclusion of BST lag 3 in our model. This choice is also substantiated by the understanding that changes in species abundance in response to temperature variations are not typically observable within the same or the subsequent year. This delay is due to the necessary growth period organisms require before they can be effectively captured in nets, considering each species’ minimum size of retention. The lag 3 thus reflects a realistic temporal frame to observe the impacts of temperature on species abundance, acknowledging the biological growth cycles intrinsic to the species under study. In general, the significant effect of BST suggests a positive relationship between environmental temperature variations and change in MTC.
Understanding the impact of environmental change on marine organisms necessitates a comprehensive grasp of their proximity to thermal limits and their capacity to adapt to rising habitat temperatures (Stillman 2003; Deutsch et al. 2008; Nguyen et al. 2011). Many marine species, including ectotherms like fish, crustaceans, and molluscs, operate near their upper thermal tolerance, making them highly susceptible to even minor temperature increases (Helmuth et al. 2005; Harley et al. 2006). These changes can significantly affect their survival, adaptation abilities, biodiversity, and community structure (Pörtner and Farrell 2008; Doney et al. 2012).
Elevated temperatures accelerate metabolic processes in ectotherms, increasing energy demands for basic functions and potentially leading to unsustainable metabolic rates and death (Pörtner and Farrell 2008; Sunday et al. 2012; Somero 2012). Additionally, higher temperatures decrease seawater’s dissolved oxygen, causing hypoxia or anoxia, detrimental to oxygen-dependent marine life (Duarte et al. 2012). Physiological stress from temperature rise triggers various organism responses, including increased heart rate, hormonal changes, and reduced immunity, leading to disease and mortality. Moreover, temperature changes affect cellular membrane integrity and disrupt internal processes like hydration, acid–base balance, and ion regulation (Duarte et al. 2012). Consequently, rising temperatures profoundly impact the health and viability of marine organisms, especially ectotherms, by altering their metabolism, oxygen availability, physiological stability, cellular integrity, and internal homeostasis.
Considering the estimated posterior mode of the coefficient of BST, a one-degree increase of BST results in an increase of 0.27 °C of MTC, on average. The value, being less than 1, indicates that communities inhabiting or projected to inhabit warmer waters are situated further from their thermal optimum. This disparity may intensify in the future. Numerous studies have demonstrated that marine communities thriving in warmer environments often experience a greater deviation from their optimal temperature range compared to those residing in colder waters. Sunday et al. (2015) analyzed the thermal physiology of 457 marine species from around the world and found that the thermal niche of many species is closer to their upper thermal limit in warmer regions. This means that in warmer regions, species are often living closer to their upper thermal limits and may be more vulnerable to the impacts of warming, such as reduced metabolic rates, reduced growth, and increased susceptibility to disease and predation. Additionally, even a small increase in temperature could have significant effect on their physiology and survival. Sorte et al. (2010) found that species living in areas where temperature varies less throughout the year have narrower thermal niches and may be more vulnerable to warming than species living in areas with more variable temperatures. This is because species in areas with more variable temperatures may have greater physiological plasticity and be better able to cope with changing temperatures. These findings suggest that species living in warmer waters may be more vulnerable to the impacts of warming due to their proximity to their thermal limits. The potential impacts of warming on marine species could have significant consequences for the structure and functioning of marine ecosystems, and for the services they provide to human societies (IPCC 2022).
There is evidence that the cause and effect relationship in marine communities can have a temporal lag of several years. Poloczanska et al. (2013) found that the timing of impacts of climate change on marine ecosystems can vary widely, with some species responding to changes in temperature and other environmental variables much more rapidly than others. This lag can occur because species may respond differently to environmental change, or because changes in environmental variables may not immediately translate into changes in population dynamics. Understanding these lag effects is important for predicting the long-term impacts of environmental change on marine ecosystems and for developing effective conservation strategies.
Temporally delayed relationships in marine communities can be explained by both physiological and ecological factors. Physiologically, some species may have mechanisms to cope with environmental change in the short term, but may not be able to sustain these mechanisms over the long term. Ecologically, some species may be more resilient or resistant to environmental change than others (Chevin et al. 2010). Resilient species may be able to recover quickly from disturbances, while resistant species may be less affected by disturbances in the first place. In contrast, species that are less resilient or resistant may experience population sudden declines or extinctions in response to environmental change. The interactions between species in a community can also influence the lag effects of environmental change. For example, a species may experience a decline in response to environmental change if it relies on another species that is also declining. This indirect effect can compound the lag effects of environmental change and make it more difficult to predict the long-term impacts on marine communities (Stachowicz et al. 2007). Overall, understanding the temporal lag in the response of marine communities to environmental change is critical for predicting the long-term impacts of environmental change on marine ecosystems and for developing effective conservation strategies. This requires a comprehensive understanding of the physiological and ecological factors that underlie the response of marine communities to environmental change, as well as the complex interactions between species in a community (Poloczanska et al. 2013).
Tsikliras and Stergiou (2014) found a time lag towards temperature variations, only for the eastern Mediterranean communities, while for those of the central Mediterranean they did not found a time lag, but a direct relationship. On the other hand, after 10 years from Tsikliras and Stergiou (2014), our study highlights a possible time lag also for the central Mediterranean communities. This could be explained by two possible hypotheses: first, central Mediterranean communities over time adapted to the changes, acquiring a certain resilience and resistance; second, thermophilic species are increasing in our communities, which are more resistant to change.
Figure 3 shows the estimated overall trend of the MTC, according to each level of depth, which was computed averaging, for each year, the posterior means of the MTC and the upper and lower bounds of their credibility intervals. To evaluate the reliability of the overall trend estimation, a retrospective analysis has been done by removing 1 to 4 years from the end of the study period, to see how much the trend estimate was affected by that. Online Fig. A9 shows a visual comparison of the estimated curves, including that of the model estimated with the complete dataset (0 years removed), from which it can be seen that trend estimations are fairly stable. Figure 4 shows the posterior means of the predicted values of the MTC across the study area (upper and lower bounds of the corresponding credible intervals are represented in Online Figs. A7 and A6, respectively). Also, to highlight both the direction and the rate of the change of the MTC level, the difference between model predictions at t and \(t-1\) time values has been computed for each time point, across the study area (Fig. 5). In particular, the MTC increase that began in 2002 become faster from 2005 to 2006; then, a quite rapid decrease happened between 2010 and 2012. The MTC does not appear to increase linearly; instead, it is characterized by step-like changes (Fig. 3 - low depth) because the distribution of marine species is not uniform in the ocean (Fig. 4), and their spatial distribution may vary non-linearly with climate change. The warming of the oceans can lead to the redistribution of marine species, as they move away from their original distribution areas in search of cooler waters or adapt to new climatic conditions. This can lead to a step-like dynamics of the MTC, in which the average temperature of the catches remains relatively stable for a certain period of time and then increases sharply due to the redistribution of marine species.
Some marine species may be able to migrate to deeper waters in response to warming temperatures, although the ability of species to do so is complex and dependent on several factors. Pinsky et al. (2013) found that some species of fish in the North Atlantic were shifting their distributions to deeper waters as a response to warming temperatures, but the rate and extent of these shifts were dependent on several factors including the physiological tolerances of the species, the depth and distribution of suitable habitats, and the rate and extent of environmental change.
However, not all species may be able to migrate to deeper waters as a response to warming temperatures. The availability of suitable habitats, competition, predation, and resource availability can all constrain the ability of species to migrate to deeper waters. Additionally, the loss of species from shallower waters may have cascading effects on food webs and ecosystem processes, while the influx of new species to deeper waters may have similar effects on those ecosystems.
The estimated overall trend of the MTC level, as is shown in Fig. 3, suggests a clear increase starting in 2003 in shallow-water area, but not in the medium and deep strata. The observed MTC increase suggests an alteration in the relative catch proportions of species; the thermophilic species (those that prefer warmer temperatures) increased in proportion in the catches over the time series, while psychrophilous (those that prefer colder temperatures) decreased (until 2015). Such change could be due to the displacement of the thermophilous species to a higher latitude and the shift of the psychrophilous species in mean latitude or depth or in both.
The ability of marine species to migrate to deeper waters as a response to warming temperatures has implications for the structure and function of marine ecosystems. It is therefore important to understand the factors that influence the ability of species to migrate to deeper waters and the potential consequences of these migrations for marine ecosystems.
Other factors such as over-fishing and contamination can also influence the distribution of marine species and the dynamics of the MTC. For example, a reduction in the populations of a particular fish species due to over-fishing can lead to a reduction of contribution in the MTC for that species.
The use of deep-sea environments as “refugia” (or protected areas) to protect marine biodiversity from the impacts of climate change has been proposed in some studies. The idea is that deep-sea environments may provide a refuge for species that are unable to cope with the changing environmental conditions at shallower depths, due to their relative stability in environmental conditions.
For example, Levin et al. (2019) proposed that deep-sea habitats may provide refuge for some species that are at risk from the impacts of climate change. The study suggested that deep-sea habitats, which are often characterized by relatively stable environmental conditions, could act as a “lifeboat” for some species, allowing them to persist in the face of warming and other environmental stressors.
However, the idea of using deep-sea environments as protected areas is not without challenges. One challenge is that deep-sea habitats are often poorly known and difficult to study, making it difficult to assess their potential as refugia. Additionally, deep-sea ecosystems are often subject to other anthropogenic stressors, such as deep-sea mining and over-fishing, which can have negative impacts on biodiversity.
Furthermore, it is important to consider the potential consequences of using deep-sea environments as refugia, such as the displacement of existing deep-sea species and potential impacts on deep-sea ecosystems. Additionally, while deep-sea habitats may provide refuge for some species, they may not be able to support entire communities, and there may be limited opportunities for connectivity between deep-sea protected areas and shallower ecosystems.
In summary, the use of deep-sea environments as refugia to protect marine biodiversity from the impacts of climate change is a subject of ongoing research and debate. While there are potential benefits to using deep-sea habitats as refugia, it is important to consider the potential challenges and consequences associated with this approach.
The process of recovery in a marine community suffering a disturbance is complex and depends on a variety of factors. One key factor is the resilience of the community, which refers to its ability to regain suffering a disturbance. Resilience is influenced by factors such as the diversity of the community, the functional roles of different species, and the availability of propagules (Bongaerts et al. 2010). Additionally, the intensity and duration of the disturbance can affect the ability of a community to recover. Once a disturbance has ceased, the process of recovery can proceed through several stages. The first stage is the recruitment of new individuals to the community, which can occur through the growth of existing individuals or the arrival of new propagules (Connell 1978). The second stage is the reestablishment of the community structure and function, which can be influenced by the order of species arrival, species interactions, and abiotic factors (Airoldi and Beck 2007). The final stage is the stabilization of the community, which occurs when the community reaches a steady state and the species composition and function become more predictable (Gorman and Connell 2009). Overall, the process of recovery in a marine community following a disturbance is complex and depends on a variety of factors, including resilience, the intensity and duration of the disturbance, and the stages of the recovery process. Understanding these factors is critical for predicting the resilience of marine communities to disturbances and for developing effective conservation strategies.
Refugia, or protected areas, are important for marine conservation and for the recovery of communities that have been impacted by disturbances (es. over-fishing, habitat destruction). These disturbances can cause a reduction in biodiversity, a decrease in vegetation cover, and a change in species composition. Refugia can help to mitigate the effects of these disturbances by providing a protected environment for marine species where organisms can find refuge and reproduce, ensuring the survival of species and the restoration of populations. Moreover, protected areas contribute to the re-establishment of ecological connections between different marine communities. This fosters the dispersal of species and facilitates the repopulation of adjacent areas. The establishment of refugia can take various forms, including marine protected areas, reserves, sanctuaries, fishing exclusion zones, and other initiatives aimed at safeguarding marine habitats. When properly designed and managed, these protected zones are crucial for the conservation and recovery of marine biodiversity inside (Sala and Giakoumi 2018) and in the adjacent fished areas (Di Lorenzo et al. 2016, 2020). In the global warming context, the MPAs are recognized to be important in protecting marine species (Frid et al. 2023).
The MTC significantly decreases with depth, as expected (estimated posterior mode of the coefficients for medium and low depth are 1.07 and 1.55, respectively, with baseline category being “high depth”): regions characterized by shallow waters are, in general, inhabited by species with higher preferred temperature. Overall, the decrease in MTC with depth highlights the importance of considering depth as a factor in fisheries management and conservation. Understanding the factors that contribute to this pattern can inform the development of more effective management strategies that account for the unique characteristics of different fish communities at different depths.
5 Conclusions
In this paper, we analysed the effects of environmental temperature on the MTC of demersal fish communities in the central Mediterranean Sea from 1995 to 2018, using the INLA-SPDE modelling approach. The proposed model allowed us to quantify the effect of ambient temperature (BST) and depth on the MTC, as well as to describe the spatio-temporal evolution of the MTC in the study area.
The study emphasises the vulnerability of ectothermic organisms such as fish, crustaceans and molluscs to even slight increases in temperature, as they operate near the upper limits of thermal tolerance. Increased temperature accelerates metabolic processes, potentially leading to unsustainable rates, oxygen depletion in the water, physiological stress and ultimately higher mortality rates. A one-degree increase in BST translates into an average 0.27 °C increase in MTC, indicating a shift away from optimal temperature conditions for marine communities. The change in MTC patterns in the study area, particularly since 2003, reflects a shift towards thermophilic species and a decline in psychrophilic species, indicative of a redistribution due to warming waters. The potential of deep-water environments as refugia for species affected by climate change was also explored. Although deepwater habitats may offer stable conditions, there are challenges, including the unknowns of these ecosystems and the impact of human activities such as over-fishing.
Our results highlight the importance of considering depth in fisheries management and conservation. The significant decrease in MTC as depth increases underlines the need for customised strategies to protect different fish communities. Refugia or protected areas are crucial to mitigate disturbance and promote the recovery and conservation of biodiversity, especially in the context of global warming.
This study, therefore, contributes to the understanding of the response of marine ecosystems to environmental changes and the development of effective conservation strategies. Overall, these results emphasise the need for continuous monitoring of marine communities and their responses to climate change, as well as the development of adaptive management strategies that take into account the complex and dynamic nature of these systems.
References
Abrahamsen P (1997) A review of Gaussian random fields and correlation functions. Norsk Regnesentral/Norwegian Computing Center Oslo
Airoldi L, Beck MW (2007) Loss, status and trends for coastal marine habitats of Europe. Oceanogr Mar Biol Annu Rev 45:345–405
Banerjee S, Carlin BP, Gelfand AE (2003) Hierarchical modeling and analysis for spatial data. Chapman & Hall/CRC, New York
Baptista V, Ullah H, Teixeira CM, Range P, Erzini K, Leitão F (2014) Influence of environmental variables and fishing pressure on bivalve fisheries in an inshore lagoon and adjacent nearshore coastal area. Estuaries Coasts 37(1):191–205
Bertrand JA, Gil De Sola L, Papaconstantinou C, Relini G, Souplet A (2002) The general specifications of the medits surveys. Sci Mar 66(S2):9–17
Bongaerts P, Riginos C, Ridgway T, Sampayo EM, van Oppen MJ, Englebert N, Vermeulen F, Hoegh-Guldberg O (2010) Genetic divergence across habitats in the widespread coral seriatopora hystrix and its associated symbiodinium. PLoS ONE 5(5):e10871
Brown JH, Gillooly JF, Allen AP, Savage VM, West GB (2004) Toward a metabolic theory of ecology. Ecology 85(7):1771–1789
Chen Z, Hu C, Muller-Karger FE, Luther ME (2010) Short-term variability of suspended sediment and phytoplankton in Tampa bay, Florida: observations from a coastal oceanographic tower and ocean color satellites. Estuar Coast Shelf Sci 89:62–72
Cheung WW, Lam VW, Sarmiento JL, Kearney K, Watson R, Zeller D, Pauly D (2010) Large-scale redistribution of maximum fisheries catch potential in the global ocean under climate change. Glob Change Biol 16(1):24–35
Cheung W, Watson R, Pauly D (2013) Signature of ocean warming in global fisheries catch. Nature 497(7449):365–368
Chevin LM, Lande R, Mace GM (2010) Adaptation, plasticity, and extinction in a changing environment: towards a predictive theory. PLoS Biol 8(4):e1000357
Colloca F, Cardinale M, Maynou F, Giannoulaki M, Scarcella G, Jenko K, Bellido JM, Fiorentino F (2013) Rebuilding Mediterranean fisheries: a new paradigm for ecological sustainability. Fish Fish 14(1):89–109. https://doi.org/10.1111/j.1467-2979.2011.00453.x
Connell JH (1978) Diversity in tropical rain forests and coral reefs. Science 199(4335):1302–1310
Consoli P, Esposito V, Battaglia P, Altobelli C, Perzia P, Romeo T, Canese S, Andaloro F (2016) Fish distribution and habitat complexity on banks of the strait of Sicily (central Mediterranean sea) from remotely-operated vehicle (Rov) explorations. PLoS ONE 11(12):e0167809
Cressie N (1993) Statistics for spatial data. Wiley, Hoboken
Cressie N, Wickle C (2011) Statistics for spatiotemporal data. Wiley, New York
Deutsch CA, Tewksbury JJ, Huey RB, Sheldon KS, Ghalambor CK, Haak DC, Martin PR (2008) Impacts of climate warming on terrestrial ectotherms across latitude. Proc Natl Acad Sci 105(18):6668–6672
Di Lorenzo M, Claudet J, Guidetti P (2016) Spillover from marine protected areas to adjacent fisheries has an ecological and a fishery component. J Nat Conserv 32:62–66
Di Lorenzo M, Guidetti P, Di Franco A, Calò A, Claudet J (2020) Assessing spillover from marine protected areas and its drivers: a meta-analytical approach. Fish Fish 21(5):906–915
Di Lorenzo M, Sinerchia M, Colloca F (2018) The north sector of the strait of Sicily: a priority area for conservation in the Mediterranean Sea. Hydrobiologia 821(1):235–253
Doney SC, Ruckelshaus M, Duffy JE, Barry JP, Chan F et al (2012) Climate change impacts on marine ecosystems. Ann Rev Mar Sci 4:11–37
Duarte CM, Lenton TM, Wadhams P, Wassmann P (2012) Abrupt climate change in the arctic. Nat Clim Chang 2(2):60–62
Dulvy NK, Rogers SI, Jennings S, Stelzenmüller V, Dye SR, Skjoldal HR (2008) Climate change and deepening of the north sea fish assemblage: a biotic indicator of warming seas. J Appl Ecol 45(4):1029–1039
Fortibuoni T, Aldighieri F, Giovanardi O, Pranovi F, Zucchetta M (2015) Climate impact on Italian fisheries (Mediterranean sea). Reg Environ Change 15:931–937
Frid O, Malamud S, Di Franco A, Guidetti P, Azzurro E, Claudet J, Micheli F, Yahel G, Sala E, Belmaker J (2023) Marine protected areas’ positive effect on fish biomass persists across the steep climatic gradient of the Mediterranean sea. J Appl Ecol 00:1–12. https://doi.org/10.1111/1365-2664.14352
Gillooly JF, Brown JH, West GB, Savage VM, Charnov EL (2001) Effects of size and temperature on metabolic rate. Science 293(5538):2248–2251
Gorman D, Connell SD (2009) Recovering subtidal forests in human-dominated landscapes. J Appl Ecol 46(6):1258–1265
Harley CDG, Randall Hughes A, Hultgren KM, Miner BG, Sorte CJB et al (2006) The impacts of climate change in coastal marine systems. Ecol Lett 9(2):228–241
Hastie T, Tibshirani R, Friedman JH, Friedman JH (2009) The elements of statistical learning: data mining, inference and prediction. Springer
Helmuth B, Kingsolver JG, Carrington E (2005) Biophysics, physiological ecology, and climate change: does mechanism matter? Annu Rev Physiol 67:177–201
Hoegh-Guldberg O, Bruno JF (2010) The impact of climate change on the world’s marine ecosystems. Science 328(5985):1523–1528
IPCC (2022) Climate change 2022: impacts, adaptation, and vulnerability. Cambridge University Press, Cambridge and New York
Keskin C, Pauly D (2014) Changes in the ‘mean temperature of the catch’: application of a new concept to the North-Eastern Aegean Sea. Acta Adriat 55(2):213–218
Krainski ET, Gómez-Rubio V, Bakka H, Lenzi A, Castro-Camilo D, Simpson D, Lindgren F, Rue H (2019) Advanced spatial modeling with stochastic partial differential equations using R and INLA. CRC Press/Taylor and Francis Group, Boca Raton
Legendre P, Legendre L (1998) Numerical ecology. Elsevier
Leitão F, Alms V, Erzini K (2014) A multi-model approach to evaluate the role of environmental variability and fishing pressure in sardine fisheries. J Mar Syst 139:128–138
Levin LA, Bett BJ, Gates AR, Heimbach P, Howe BM, Janssen F, McCurdy A, Ruhl HA, Snelgrove PV, Stocks KI et al (2019) Global observing needs in the deep ocean. Front Mar Sci 6:241
Levitus S, Antonov JI, Boyer TP, Stephens C (2000) Warming of the world ocean. Science 287(5461):2225–2229
Li M, Zhong L, Harding LW (2009) Sensitivity of plankton biomass and productivity to variations in physical forcing and biological parameters in chesapeake bay. J Mar Res 67:667–700
Lindgren F, Rue H (2015) Bayesian spatial modelling with R-INLA. J Stat Softw 63(19):1–25
Lindgren F, Rue H, Lindstrom J (2011) An explicit link between Gaussian fields and Gaussian Markov random fields: the stochastic partial differential equation approach. J R Stat Soc Ser B 73:423–498
Maharaj RR, Lam VW, Pauly D, Cheung WW (2018) Regional variability in the sensitivity of Caribbean reef fish assemblages to ocean warming. Mar Ecol Prog Ser 590:201–209
Matérn B (1960) Stochastic models and their application to some problems in forest surveys. Stockholm
Nguyen KDT, Morley SA, Lai CH, Clark MS, Tan KS et al (2011) Upper temperature limits of tropical marine ectotherms: global warming implications. PLoS ONE 6(12):e29340
Olden JD, Neff BD (2001) Cross correlation bias in lag analysis of aquatic time series. Mar Biol 138:1063–1070
Parraga DP, Cubillos LA, Correa-Ramirez MA (2010) Spatiotemporal variations of the catch per unit effort in the coastal small-scale fishery of snapper lutjanus synagris, of the Colombian Caribbean and their relationship with environmental variables. Revista de Biologia Marina y Oceanografia 45:77–88
Perry AL, Low PJ, Ellis JR, Reynolds JD (2005) Climate change and distribution shifts in marine fishes. Science 308(5730):1912–1915
Pinsky ML, Worm B, Fogarty MJ, Sarmiento JL, Levin SA (2013) Marine taxa track local climate velocities. Science 341(6151):1239–1242
Poloczanska E, Brown C, Sydeman W et al (2013) Global imprint of climate change on marine life. Nat Clim Chang 3:919–925
Pörtner HO, Farrell AP (2008) Physiology and climate change. Science 322(5902):690–692
Qiu Y, Lin Z, Wang Y (2010) Responses of fish production to fishing and climate variability in the Northern South China sea. Prog Oceanogr 85:197–212
Rue H, Held L (2005) Gaussian Markov random fields: theory and applications. Chapman & Hall, Boca Raton
Rue H, Martino S, Chopin N (2009) Approximate Bayesian inference for latent Gaussian models by using integrated nested Laplace approximations (with discussion). J R Stat Soc Ser B 71:319–392
Rue H, Riebler A, Sorbye SH, Illian JB, Simpson DP, Lindgren F (2017) Bayesian computing with INLA: a review. Annu Rev Statist App 4:395–421
Sala E, Giakoumi S (2018) No-take marine reserves are the most effective protected areas in the ocean. ICES J Mar Sci 75(3):1166–1168
Simpson D, Illian JB, Lindgren F, Sørbye SH, Rue H (2016) Going off grid: computationally efficient inference for Log-Gaussian Cox processes. Biometrika 103:49–70
Somero GN (2012) The physiology of global change: linking patterns to mechanisms. Ann Rev Mar Sci 4:39–61
Sorte CJ, Williams SL, Carlton JT (2010) Marine range shifts and species introductions: comparative spread rates and community impacts. Glob Ecol Biogeogr 19(3):303–316
Spiegelhalter DJ, Best NG, Carlin BP, Van Der Linde A (2002) Bayesian measures of model complexity and fit. J R Stat Soc Ser B Stat Methodol 64(4):583–639
Stachowicz JJ, Bruno JF, Duffy JE (2007) Understanding the effects of marine biodiversity on communities and ecosystems. Annu Rev Ecol Evol Syst 38:739–766
Stillman JH (2003) Acclimation capacity underlies susceptibility to climate change. Science 301(5635):65–65
Sunday JM, Bates AE, Dulvy NK (2012) Thermal tolerance and the global redistribution of animals. Nat Clim Chang 2(9):686–690
Sunday JM, Pecl GT, Frusher S, Hobday AJ, Hill N, Holbrook NJ, Edgar GJ, Stuart-Smith R, Barrett N, Wernberg T et al (2015) Species traits and climate velocity explain geographic range shifts in an ocean-warming hotspot. Ecol Lett 18(9):944–953
Tsikliras AC, Peristeraki P, Tserpes G, Stergiou KI (2015) Mean temperature of the catch (MTC) in the Greek Seas based on landings and survey data. Front Mar Sci 2:23
Tsikliras AC, Stergiou KI (2014) Size at maturity of Mediterranean marine fishes. Rev Fish Biol Fisheries 24:219–268
Valente S, Moro S, Di Lorenzo M, Milisenda G, Maiorano L, Colloca F (2023) Mediterranean fish communities are struggling to adapt to global warming. Mar Environ Res 191:106176
Vidal J, Moreno-Ostos E, Escot C, Quesada R, Rueda F (2010) The effects of diel changes in circulation and mixing on the longitudinal distribution of phytoplankton in a canyon-shaped mediterranean reservoir. Freshw Biol 55:1945–1957
Von Biela VR, Zimmerman CE, Moulton LL (2011) Long-term increases in young-of-the-year growth of Arctic cisco Coregonus autumnal is and environmental influences. J Fish Biol 78(1):39–56
Wang X, Yue YR, Faraway JJ (2018) Bayesian regression modeling with INLA. CRC Press
Funding
Open access funding provided by Università degli Studi di Palermo within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Luiz Duczmal.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rubino, C., Adelfio, G., Abbruzzo, A. et al. Exploring the effects of temperature on demersal fish communities in the Central Mediterranean Sea using INLA-SPDE modeling approach. Environ Ecol Stat (2024). https://doi.org/10.1007/s10651-024-00609-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10651-024-00609-7