Abstract
Mercury (Hg) pollution is an environmental problem that adversely affects human and ecosystem health at local, regional, and global scales—including within New York State. More than two-thirds of the Hg currently released to the environment originates, either directly or indirectly, from human activities. Since the early 1800s, global atmospheric Hg concentrations have increased by three- to eight-fold over natural levels. In the U.S., atmospheric emissions and point-source releases to waterways increased following industrialization into the mid-1980s. Since then, water discharges have largely been curtailed. As a result, Hg emissions, atmospheric concentrations, and deposition over the past few decades have declined across the eastern U.S. Despite these decreases, Hg pollution persists. To inform policy efforts and to advance public understanding, the New York State Energy Research and Development Authority (NYSERDA) sponsored a scientific synthesis of information on Hg in New York State. This effort includes 23 papers focused on Hg in atmospheric deposition, water, fish, and wildlife published in Ecotoxicology. New York State experiences Hg contamination largely due to atmospheric deposition. Some landscapes are inherently sensitive to Hg inputs driven by the transport of inorganic Hg to zones of methylation, the conversion of inorganic Hg to methylmercury, and the bioaccumulation and biomagnification along food webs. Mercury concentrations exceed human and ecological risk thresholds in many areas of New York State, particularly the Adirondacks, Catskills, and parts of Long Island. Mercury concentrations in some biota have declined in the Eastern Great Lakes Lowlands and the Northeastern Highlands over the last four decades, concurrent with decreases in water releases and air emissions from regional and U.S. sources. However, widespread changes have not occurred in other ecoregions of New York State. While the timing and magnitude of the response of Hg levels in biota varies, policies expected to further diminish Hg emissions should continue to decrease Hg concentrations in food webs, yielding benefits to the fish, wildlife, and people of New York State. Anticipated improvements in the Hg status of aquatic ecosystems are likely to be greatest for inland surface waters and should be roughly proportional to declines in atmospheric Hg deposition. Efforts that advance recovery from Hg pollution in recent years have yielded significant progress, but Hg remains a pollutant of concern. Indeed, due to this extensive compilation of Hg observations in biota, it appears that the extent and intensity of the contamination on the New York landscape and waterscape is greater than previously recognized. Understanding the extent of Hg contamination and recovery following decreases in atmospheric Hg deposition will require further study, underscoring the need to continue existing monitoring efforts.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mercury pollution is an environmental problem with local, regional, and global dimensions that adversely affects human and ecosystem health—including that in New York State, USA. Mercury (Hg) is a naturally occurring element that is emitted from volcanoes and released by natural processes such as soil weathering (Driscoll et al. 2013). However, more than two-thirds of the Hg currently released to the environment globally originates, either directly or indirectly, from human activities (Amos et al. 2013). For more than 200 years, Hg has been released into the atmosphere and waterways in New York State by human activities, including emissions from fossil fuel combustion, waste incineration, metal smelting, and chlorine production, and discharges to surface waters from domestic and industrial wastewater. Since the early 1800s, global releases have increased atmospheric Hg concentrations by three- to eight-fold above natural levels (Streets et al. 2017; UN Environment 2019).
Mercury has long been recognized as an important environmental problem in New York State (Bloomfield et al. 1980). Factors that contribute to this condition include: the location downwind of the many coal-fired power plants in the Ohio River Valley; extensive forested lands particularly in the Adirondacks and Catskills that enhance deposition; an abundance of wetlands that facilitate methylation; and numerous streams, rivers, lakes, and estuaries with extensive fish populations and other wildlife that are exposed to methylmercury (MeHg) through trophic transfer (Driscoll et al. 2007). As a result, there are many Hg-related fish consumption advisories for specific inland freshwaters, the two Great Lakes (Erie, Ontario), and coastal areas of New York State, as well as blanket advisories for the Adirondacks and Catskills (https://www.health.ny.gov/environmental/outdoors/fish/health_advisories/). Despite these challenges, efforts are underway to curb Hg pollution.
Under the Great Lakes Water Quality Agreement, Environment Canada and the U.S. Environmental Protection Agency (US EPA) signed the Great Lakes Binational Toxics Strategy in 1997 calling for virtual elimination of Hg emissions originating from human sources in the Great Lakes region (US EPA 1997). The Great Lakes Regional Collaboration built on this effort and in 2010 produced the Great Lakes Mercury Emission Reduction Strategy with recommendations for decreasing emissions from the largest remaining sources in the basin. The Mercury Air Toxics Standards (MATS) rule went into effect in 2015 with a goal of achieving 91% reductions in Hg emissions from electric utilities (https://www.epa.gov/mats). At the global scale, the Minamata Convention on Mercury entered into force in August 2017, which includes the United States as a ratified Party for this international treaty (Evers et al. 2016).
To advance scientific and public understanding and to inform policy efforts, the New York State Energy Research and Development Authority (NYSERDA) sponsored a scientific synthesis on Hg in atmospheric deposition, water, invertebrates, fish, and wildlife in New York State. The result of this scientific collaboration is a series of 23 papers published in Ecotoxicology (Table 1). The findings from these studies are summarized here in the context of an extensive statewide database of Hg concentrations in atmospheric deposition, surface waters, and in a broad array of biota including fish, birds, invertebrates, and others that were compiled as part of the scientific synthesis. The database is presented in summarized form and results from the 23 papers are integrated into this summary article as we answer a series of questions about Hg in New York State including the concentrations that occur and associated risks, spatial and temporal patterns, and policies that are addressing exposure risks.
Study area
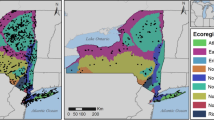
The USEPA has identified 104 ecoregions across the U.S. In New York, USEPA Level III Ecoregions divide the state into similar lands based on patterns in the mosaic of biotic, abiotic, aquatic, and terrestrial ecosystem components (Fig. 1). These ecoregions provide a spatial framework to evaluate Hg exposure patterns of fish and wildlife that are adapted to these different environments (Fig. 2).
Ecoregions across New York State, United States (Data obtained at: https://www.epa.gov/eco-research/level-iii-and-iv-ecoregions-continental-united-states)
Land cover types of New York State. The state has more than 7600 freshwater lakes, ponds, and reservoirs, as well as portions of Lake Erie and Lake Ontario and over 112,000 km of rivers and streams. After the Great Lakes, New York’s largest lakes include Champlain, Oneida, Seneca, and Cayuga Lakes. Seneca and Cayuga Lakes are part of a series of long, narrow lakes known as the Finger Lakes located in central New York. The State’s largest rivers include the St. Lawrence, Susquehanna, Allegheny, Hudson, and Delaware Rivers (Data obtained at: https://www.usgs.gov/centers/eros/science/national-land-cover-database?qt-science_center_objects=0#qt-science_center_objects)
Methods
Sampling and measurement units
Observations of Hg concentrations in abiotic media (air, water) and biotic samples (invertebrates, fish, amphibians, reptiles, birds, and mammals) from New York State are available from 1969 through 2017; the total sample size within the New York State Hg database described in this paper was over 70,000 observations of Hg concentrations (including over 47,000 from biota—Table 2). These data comprise the “NYSERDA Synthesis of Environmental Mercury Loads in New York State (1969–2017)” (NYSMD), which is available at Open NY (see Acknowledgments section for URL). Mercury biota data were standardized for each major taxonomic group to a common tissue type (Table 2). For most species, total mercury (THg) was measured and predominantly occurs as MeHg, but in species where MeHg is a smaller fraction of THg, MeHg concentrations were directly measured and are identified within this paper when relevant. Mercury in biotic samples is generally reported in parts per million (ppm) of wet weight (ww).
Spatial grid system
To examine Hg patterns in biota (i.e., fish, birds) across New York, the state was divided into 1/8 by 1/8 degree grid cells, each of which represents approximately 250 square kilometers. The number in each grid cell in Fig. 3 depicts the number of wildlife species sampled for Hg. This approach was used in past assessments of effects of Hg loads including biota for the Great Lakes (Evers et al. 2011).
The data from the NYSMD were overlaid across the spatial grid system after standardization of Hg data (Fig. 3). All THg observations were first standardized to a representative tissue type for each taxonomic group. When relevant, bird observations were converted to blood THg (ppm, ww) (Eagles-Smith et al. 2009; Evers et al. 2011). All fish THg data were standardized to whole fish THg (ppm, ww) and were length-standardized to control for variation attributable to size and age using a species-specific general linear mixed model (Buck et al. 2019).
Spatial analyses
A spatial analyses was conducted to explore variation of fish Hg concentrations across the state in the context of human and wildlife health risk, with a focus on the landscape characteristics that affect spatial patterns. Two subsets of the NYSMD were created for the spatial analysis. The first included all fish data, while the second subset selected for game fish. These two datasets were then independently analyzed with a general linear mixed model fit in a Bayesian framework using packages rstan and rstanarm (Carpenter et al. 2017; Goodrich et al. 2018; Stan Development Team 2018) within the R statistical modeling environment (v 3.5.1; R Core Team 2018). Foraging guild and tissue type were fixed effects, while sampling year and species were random effects nested within grid cell as a spatial covariate. The model predicted mean THg concentrations for all fish at an omnivore or higher trophic level for each grid cell where a fish was sampled and were then mapped across New York State. See Adams et al. (2020) for further information on data compilation, standardization, and model development. To better understand the implications of these results for human health, the fish THg concentrations are presented in relation to effect level thresholds published jointly by the US Food and Drug Administration and US EPA (FDA/EPA 2019; see Table 3 for consumption guidelines).
Additionally, landscape scale variables play an important role in Hg availability to biota. Mercury concentrations in fish are driven in part by the Hg inputs (e.g., atmospheric deposition), methylation rates, and trophic level. A portion of the atmospherically deposited Hg is converted to its bioavailable form, MeHg. Landscape characteristics can amplify this conversion process to contribute to the spatial heterogeneity of Hg across the landscape. A spatial analysis of Land Use and Land Cover (LULC) data for New York State was conducted to highlight these relationships. The percent cover of forest and agriculture were extracted, for each grid cell, from the National Land Cover Database (NLCD) and, where grid cells overlapped spatially with Canada, the Land Cover of Canada database (Latifovic et al. 2017; Yang et al. 2018). A simple linear model was then constructed to examine the relationship between game fish Hg and LULC (forest and agriculture, respectively) by grid cell.
Temporal analyses
Temporal analyses were conducted to assess changes in Hg concentrations for 15 well-sampled (>10 total years of sampling, >400 total samples) indicator species (11 fish and four birds) by USEPA Level III Ecoregions in New York State. This dataset was also analyzed using a general linear mixed model with the same framework as the spatial analysis. The temporal model used tissue type as a fixed effect, while the nested effect of ecoregion, species, and year were random effects. The output of this model predicted a species-specific mean Hg concentration annually for each ecoregion, year combination sampled for that respective species (while controlling for grid-cell level spatial variation). These predicted values were then input into a series of linear models to examine species-specific trends in Hg concentrations through time for each ecoregion, species combination with more than 2 years of sampling. See Adams et al. (2020) for further information on data compilation, standardization, and model development.
Results and discussion
Measurements of environmental Hg concentrations and fluxes improve understanding of Hg dynamics and effects in the environment and help parameterize models that simulate and project fate and exposure of Hg. Through a collaborative effort, this special issue of Ecotoxicology includes 23 papers representing a wide variety of Hg studies across New York State including atmospheric deposition (one paper), surface waters (two papers), invertebrates (three papers), fish (seven papers) and birds (ten papers) (Table 1). These papers collectively characterize environmental Hg deposition and concentrations in ecosystems of New York State, evaluate patterns of methylation, determine bioaccumulation and exposure in aquatic and terrestrial food webs, and address temporal trends of Hg in the context of changes in emissions and atmospheric deposition using various biota. This work also places Hg contamination in New York State in context with other areas of the U.S (Grieb et al. 2020; Olson et al. 2020). Integrating the findings from these papers provides a robust assessment of the impact of Hg on ecosystems of New York State, as well as a broader scientific basis for policymakers and natural resource managers to make informed decisions regarding future air regulations and fish consumption advisories.
Our findings about the extent and effects of Hg pollution in New York State are described in the context of three policy-relevant questions: (1) What are the sources of Hg inputs to New York State and how do these inputs vary over space and time?; (2) What risks does mercury pollution pose to humans in New York State?; and (3) What are the spatial and temporal patterns of Hg in biota in New York State and to what extent do these reflect sensitivity to Hg inputs and changes in those inputs? Specific science-policy connections are addressed at the end of this paper.
What are the sources of mercury inputs to New York State and how do these inputs vary over space and time?
Mercury emission sources that affect New York State
Initial regulatory efforts in the 1970s focused on large industrial sources of Hg, such as chlor-alkali plants. These point sources discharged Hg directly or indirectly into the Great Lakes and their tributaries. Today, many of these sources have been reduced or eliminated, which has led to a partial recovery from point-source Hg pollution (Evers and Clair 2005; Cain et al. 2011; Drevnick et al. 2012; Todorova et al. 2014). Atmospheric emissions and deposition are currently the largest source of Hg to New York State. Stationary sources (e.g., fossil fuel burning plants and waste incinerators) emit Hg into the atmosphere as gaseous elemental Hg, gaseous oxidized Hg, or particulate bound Hg. Gaseous elemental Hg can be transported long distances (atmospheric residence time ~0.5–1 yr), while gaseous oxidized Hg and particulate bound Hg tend to be deposited near the source (hours to days). For this reason, Hg deposition to New York State can originate from local, regional, national, or global sources.
Controls on Hg emissions in the US were first initiated by designation of Hg as a hazardous air pollutant through the 1990 Amendments of the Clean Air Act (CAA). The US EPA was required to list and set emissions standards which later resulted in a finding that it was appropriate and necessary to regulate Hg emissions from coal- and oil-fired power plants and other sources such as incinerators (Milford and Pienciak 2009). This began a regulatory process which has led to a more than four-fold decline in U.S. anthropogenic Hg emissions between 1990 and 2017 (Olson et al. 2020). During 1990 to 2005, the largest decreases in Hg emissions were from hospital and municipal incinerators (96–99% decrease) and chlor-alkali facilities (97% decrease; Schmeltz et al. 2011). Mercury emissions from electric utilities have declined since the 1990s due to a variety of state and federal regulations, many of which affected emissions indirectly as co-benefits of controlling other pollutants such as sulfur dioxide and nitrogen oxides (Milford and Pienciak 2009; Schmeltz et al. 2011; Zhang et al. 2016) and in recent years following the implementation of the MATS rule. The average rate of decline of Hg emissions from electric utilities was −7.4 percent per year during 1996 to 2017 (Olson et al. 2020). In the U.S., approximately 52 tons of Hg were annually emitted from anthropogenic sources in 2014 (Bourtsalas and Themelis 2019), decreasing to 33 tons in 2017. Emissions of Hg from coal-fired power plants have decreased to the point that they are no longer the largest source of anthropogenic atmospheric Hg emissions in the U.S., being replaced by industrial processes (Fig. 4). There are major and minor sources of anthropogenic Hg emission sources across New York State, the largest Hg emission source is “other categories” which include nonpoint emissions and point source emissions associated with various industries (Fig. 5). In contrast to declines in New York State and the U.S., inventories suggest that global anthropogenic Hg emissions have increased in recent years (Streets et al. 2019; UN Environment 2019). Asia continues to be a dominant contributor to Hg emissions globally, due largely to expanding energy production from coal-fired power plants and gold mining.
US EPA National Emissions Inventory (NEI) estimates of Hg emitted to the air by major sources in the United States from 1990 to 2017. Coal-fired power plants remain the largest source of Hg emissions in the US (USEPA 2020)
US EPA NEI estimates of Hg emitted to the air by major source in New York State from 1999 to 2017 (USEPA 2020)
Source attribution of Hg has been conducted for New York State and the Northeast. Lin et al. (2012) conducted source apportionment of Hg emissions for the contiguous U.S. using the Community Multiscale Air Quality (CMAQ) model. In New York and New England, relatively high contributions of Hg were derived from electrical generating units and waste incineration. Sources outside the U.S. also contributed to Hg deposition in New York and New England but the fractions were smaller than in many other regions of the U.S. Choi et al. (2008) conducted source contribution analysis for forms of atmospheric Hg in the Adirondacks observing that Pennsylvania, West Virginia, Ohio, Kentucky, Texas, Indiana, and Missouri were important contributing areas.
Spatial and temporal patterns in atmospheric Hg concentrations and deposition
Ye et al. (2020) modeled Hg deposition across New York State, using CMAQ with state-of-the-science air chemistry algorithms. They examined the contribution of local emissions and the role of environmental variables to spatial patterns of Hg deposition (Fig. 6). Meteorological conditions and forest canopy characteristics were the most important factors affecting spatial patterns in Hg wet and dry deposition, respectively.
Simulated spatial patterns of dry and wet Hg deposition across New York State based on Ye et al. (2020) at a 1/8 by 1/8 degree grid scale. Patterns of (a) dry and (b) wet Hg deposition vary considerably across the state due to emissions point sources, precipitation, wind speed, heat flux, and land cover. The z-axes for dry and wet deposition are shown on different scales
Dry deposition: Local, anthropogenic Hg emissions sources increase deposition rates over water and land within 50 km of the point source. For example, emission sources near Rochester, in Manhattan, and on Long Island contribute to elevated dry Hg deposition in those areas (Fig. 6a). Environmental factors such as wind speed and heat flux (changes in temperature across the surface of water and land) drive spatial patterns of deposition in aquatic habitats, while increased canopy cover associated with forested lands lead to greater deposition in terrestrial landscapes.
Wet deposition: The amount of precipitation is an important driver of spatial variation in wet Hg deposition to aquatic and terrestrial habitats. Higher levels of precipitation downwind of Lake Erie and Lake Ontario lead to increased Hg deposition in the central and western regions of the state (Fig. 6b).
In the U.S., concentrations of atmospheric Hg species are monitored by the Atmospheric Mercury Network (AMNet; http://nadp.slh.wisc.edu/amnet/). In New York State, there are three AMNet sites of the National Atmospheric Deposition Program. Olson et al. (2020) examined temporal trends in atmospheric Hg species generally finding decreases in concentrations of gaseous elemental Hg (7a) and gaseous oxidized Hg (7b) in the Northeast from 2008 to 2017, which are consistent with regional and national decreases in emissions. Trends for particulate bound Hg are more varied with some sites in New York State showing increases in concentrations (7c), which may be caused by wood burning in winter (Zhou et al. 2017) (Fig. 7).
Long-term temporal trends in concentrations of (a) gaseous elemental Hg, (b) gaseous oxidized Hg, and (c) particulate bound Hg for the northeastern U.S. A blue symbol indicates a decreasing trend and a red symbol shows an increasing trend. Large symbols are indicative of a significant trend. Modified after Olson et al. (2020)
Trends in wet Hg deposition are monitored by the Mercury Deposition Network (MDN; http://nadp.slh.wisc.edu/mdn), of the National Atmospheric Deposition Program, including six sites in New York State. Olson et al. (2020) evaluated long-term temporal trends in wet Hg deposition during 1996 to 2017. They found generally consistent decreasing trends in annual volume-weighted concentrations for stations in the eastern U.S., including all of those in New York State (Fig. 8a) which is generally consistent with decreases in U.S. emissions. Trends in annual wet Hg deposition are less than those of concentrations, although many sites in the Northeast show significant decreasing trends (Fig. 8b). This diminished trend is due to considerable year-to-year variability in precipitation quantity and a general pattern of increasing precipitation quantity in the Northeast (Fig. 8c).
Long-term trends in (a) annual volume-weighted concentrations of Hg in wet deposition, (b) annual wet Hg deposition and (c) annual precipitation quantity for the northeastern U.S. from the Mercury Deposition Network. A blue symbol indicates a decreasing trend and a red symbol shows an increasing trend. Large symbols are indicative of a significant trend. Modified after Olson et al. (2020)
Landscape factors have been shown to exert important controls on Hg deposition. In particular, the forest canopy mediates land-atmosphere exchange of Hg. Elemental Hg enters the stomata of leaves and is later deposited to soil as litterfall. Ionic Hg can be adsorbed to the surface of leaves and needles and washed off during subsequent rain events and deposited as throughfall. In New York State, studies have shown that in hardwood stands litterfall is the largest pathway of Hg deposition to the forest floor (Blackwell et al. 2014). However, Hg deposition is typically greater in coniferous stands than in hardwoods because of the persistence of needles throughout the year and the high leaf area index of the coniferous canopy, and throughfall is the dominant pathway of Hg deposition (Blackwell et al. 2014). Finally, Hg deposition increases with elevation due to increases in precipitation quantity and shifts in vegetation (Blackwell and Driscoll 2015). At high elevations, cloud deposition can dominate atmospheric Hg input (Gerson et al. 2017). Soil and surface water Hg concentrations increase with elevation, likely reflecting increases in deposition (Blackwell and Driscoll 2015; Millard et al. 2020b).
What risks to humans does mercury pollution pose in New York State?
Fish are well-established bioindicators of environmental Hg loads and conditions that facilitate the transport, transformations and trophic transfer of Hg. Fish data are used to understand long-term temporal trends in Hg (Millard et al. 2020b; Riva-Murray et al. 2020a; Richter and Skinner 2020; Swinton and Nierzwicki-Bauer 2020), evaluate human health concerns (Grieb et al. 2020), link to land management and water quality (Millard et al. 2020b; Razavi et al. 2020), and inform fisheries management (Taylor et al. 2020). Fish Hg data are also used to inform policy and management in New York State.
Mercury exposure—risks to humans
Human populations most at risk of MeHg exposure include: (1) sensitive individuals (e.g., women of childbearing age, pregnant women, and children); and (2) people whose diets include large amounts of high trophic-level fish (e.g., recreational anglers and subsistence fish consumers). The greatest risks to humans from dietary uptake of MeHg are observed with high consumption levels of upper trophic level species. For example, primary consumers (e.g., shellfish such as mussels) at trophic level 2 have relatively low MeHg concentrations and are considered safe for consumption. Secondary consumers (e.g., salmon, herring) are a trophic step higher, but are also healthy choices.
For tertiary or higher consumers, which include predatory fish (e.g., bass, walleye, bluefish), MeHg concentrations can be elevated to levels that raise human health concerns. The variability of concentrations in trophic level 4 fish can be related to size and species. Therefore, large trophic level 4 fish are the best bioindicators to assess potential exposure risk of Hg to humans. In coastal waters of New York State and nearby regions, large marine predatory fish such as tuna, swordfish and shark can have elevated MeHg concentrations, frequently exceeding the no consumption limits identified by the USEPA (i.e., 0.46 ppm, ww) and the Great Lakes Consortium (i.e., 0.95 ppm, ww) (Karimi et al. 2012, 2013; Lee et al. 2016).
New York State fish consumption advisories are issued by the New York State Department of Health (DOH) and are based on a risk management approach and guidelines (Table 3). If there is no specific advice for a water body, a general statewide advisory that recommends limiting sport fish consumption to up to four meals per month applies (because fish from all waters have not been tested, and fish may contain contaminants other than those that are routinely tested). In most cases, if a water body has a specific consumption advisory for the general population (men and older women), the sensitive population (women under 50 and children under 15) are advised not to consume any fish from that water body. Women under 50 and children under 15 are also advised to avoid consuming predator fish species from all water bodies in the high Hg regions of the Catskills and Adirondacks. There are fish consumption advisories for Hg in 92 water bodies New York State, with 62 of these in the Adirondacks (https://www.health.ny.gov/environmental/outdoors/fish/health_advisories/).
Mercury Concentrations in waters of New York State
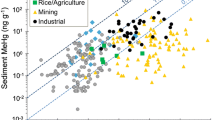
Mercury concentrations in the 15 species of fish with the highest levels in three major aquatic ecosystems of New York State vary by species (Fig. 9). Fish Hg concentrations for Lake Erie and Lake Ontario over the past three decades, remain on average above the 0.22 ppm (ww) advisory threshold suggested by the Great Lakes Consortium for 9 of the top 15 species analyzed (60%), based on the NYSMD (n = 2633; Fig. 9a). Fish in the lower Great Lakes with the highest concentrations of Hg include white bass (Morone chrysops), white perch (Morone americana), and walleye (Sander vitreus).
a Mean concentrations and standard deviation of Hg in fish of Lake Erie and Lake Ontario of New York State (n = 2633). Three vertical lines indicate consumption advisory levels. The 0.15 and 0.46 ppm vertical lines bracket the USEPA advisory levels. The mean Hg concentration of 9 of 15 species exceed the 0.22 ppm health threshold suggested by the Great Lakes Consortium (Table 3). b Mean concentrations and standard deviation of Hg in fish of inland waters of New York State (n = 21,084). Three vertical lines indicate consumption advisory levels. The 0.15 and 0.46 ppm vertical lines bracket the USEPA advisory levels. The mean Hg concentration of 11 of 15 species exceed the 0.22 ppm health threshold of the Great Lakes Consortium (Table 3). c Mean concentrations and standard deviation of Hg in fish of nearshore marine waters of New York State (n = 1204). Three vertical lines indicate consumption advisory levels. The 0.15 and 0.46 ppm vertical lines bracket the USEPA advisory levels. The mean Hg concentration of 5 of 15 species exceed the 0.22 ppm health threshold of the Great Lakes Consortium (Table 3)
There is considerable variability in fish Hg concentrations in inland waters of New York State, depending on region, species, and type of water body. Understanding this variability is important for interpreting patterns of exposure and monitoring trends for human and ecological health. Fish Hg concentrations in inland waters over the past three decades, remain on average above the 0.22 ppm (ww) advisory threshold for 11 of the top 15 species (73%) based on the NYSMD (n = 21,084; Fig. 9b). Inland water fish with the highest concentrations of Hg include walleye, smallmouth bass (Micropterus dolomieu), and white perch. In the Finger Lakes region, fish Hg concentrations of most concern for human consumption are found in walleye and largemouth bass (Micropterus salmoides) but were not correlated with aquatic invertebrate MeHg concentrations (Razavi et al. 2020). In a survey of patterns of fish Hg across New York State, Millard et al. (2020b) found the highest concentrations in the Adirondack and Catskill regions.
In coastal marine waters, where most of the human fish consumption diet originates (over 90% in North America; Sunderland et al. 2018), fish Hg concentrations exceed 0.22 ppm (ww) for 5 of the top 15 species analyzed (33%), based on the NYSMD (n = 1204; Fig. 9c). Patterns of coastal marine fish Hg are challenging to understand as sampling efforts are less robust than inland waters (Grieb et al. 2020). Chen et al. (2020) investigated the role of nutrient supply in fish Hg concentrations in coastal Long Island. In shallow coastal lagoons of Great South Bay and Jamaica Bay, they found that Hg inputs cycle through sediments which are continuously resuspended into the water column and redeposited back to sediments. They found that nutrient supply alters the uptake of MeHg at the algal base of the food chain through biodilution, while nitrogen appears to subsequently enhance trophic transfer of MeHg to prey fish.
What are the spatial and temporal patterns of mercury in biota in New York State and to what extent do these reflect sensitivity to mercury inputs and changes in those inputs?
Ecosystem sensitivity to mercury
Inorganic Hg enters ecosystems through atmospheric deposition, water releases (e.g., from wastewater treatment facilities, Glass et al. 1990), and leaching from land (e.g., from landfills and industrially contaminated sites; Kocman et al. 2017; Streets et al. 2017; Hsu-Kim et al. 2018; Obrist et al. 2018). Once in the environment, inorganic Hg can be transported to reducing zones (wetlands, sediments) and converted to MeHg by bacteria and archea (Gilmour et al. 2013a, 2013b; Podar et al. 2015). Methylmercury is toxic and can bioaccumulate at the base of the food web and biomagnify through the food chain resulting in elevated concentrations in the tissues of fish, wildlife, and humans, causing adverse health effects.
New York State and much of the Great Lakes basin is a net sink for Hg inputs (Denkenberger et al. 2012), with more Hg entering the basin through emissions and deposition than leaving through re-emission to the atmosphere or drainage losses. As a result, Hg deposited to the region accumulates in soils, and some of this legacy Hg is gradually leached to surface waters. Mercury recently deposited to the landscape tends to be more bioavailable than Hg long buried in soils and sediments (Hintelmann et al. 2002); although, Hg in soils can be mobilized by disturbances, such as storm runoff events, fires, and logging (Willacker et al. 2019; Denkenberger et al. 2020).
Mercury-sensitive areas with abundant forests receive elevated Hg inputs via throughfall and litterfall from atmospheric emissions and deposition to the forest canopy (Fig. 10). A fraction of the Hg deposited to sensitive landscapes is converted to MeHg largely in wetlands, sediments, and other favorable reducing environments. This conversion process is amplified under conditions of high organic matter, low oxygen, and low pH, that are common in northern forest landscapes (Hsu-Kim et al. 2013; Fig. 10). Sulfate has also been shown to influence MeHg formation. Methylation rates increase up to concentrations of about 20 mg/L and decline under elevated sulfate concentrations in sulfur addition experiments (Gilmour and Henry 1991). However, in natural wetland settings where atmospheric deposition is the primary source of sulfate to the landscape, sulfate availability and MeHg are strongly correlated with no limitation evident (Åkerblom et al. 2013). Conditions where low oxygen, high organic matter, and sulfate reduction occur such as in wetlands, are particularly effective at mobilizing MeHg, which may be further promoted by drying-rewetting cycles (Millard et al. 2020a; Wang et al. 2020). Furthermore, areas that supply elevated dissolved organic carbon (DOC) from decaying organic matter may facilitate the transport of inorganic and MeHg to downstream environments (Dittman et al. 2010; Burns et al. 2012; Millard et al. 2020a) and may be correlated with MeHg concentrations in macroinvertebrates (e.g., odonates; Nelson et al. 2020) and fish (Driscoll et al. 2007). Regions that have been acidified by acid deposition may be particularly sensitive to atmospheric Hg deposition, as sulfate from atmospheric deposition can serve as an important substrate for methylation (Wyn et al. 2009; Coleman Wasik et al. 2015), and Hg strongly bioaccumulates in zooplankton, fish, and loons at low pH (Driscoll et al. 2007; Yu et al. 2011). Finally, the nutrient supply and productivity of the water bodies can influence the concentration of Hg through biodilution and growth dilution (Driscoll et al. 2007, 2012; Chen et al. 2020).
In addition to variation in watershed sensitivity throughout the region, climatic variation and events can affect spatial patterns of Hg concentrations in biota. Swinton et al. (2020) described significant increases of Hg in smallmouth bass and yellow perch (Perca flavescens) on Lake Champlain and attributed to increased tributary loading of total suspended solids that resulted from a past storm runoff event.
In areas where atmospheric Hg deposition is moderate, effects on biota may be disproportionately high if conditions exist that promote Hg transport, methylation, and trophic transfer. Conversely, ecosystems with limited ability to transport inorganic Hg to zones of methylation or low methylation potential may have low levels of MeHg in biota despite considerable anthropogenic Hg contamination. The decoupling of inorganic Hg sources with MeHg production and bioaccumulation and biomagnification is evident at local (Evers et al. 2007) and landscape levels (Eagles-Smith et al. 2016). Millard et al. (2020a) found that dissolved organic matter and associated Hg was mobilized from soil to streamwater by watershed liming (calcium carbonate application). However, this enhanced transport did not result in increased MeHg concentrations in streamwater and stream macroinvertebrates due to limited zones of methylation (riparian areas, wetlands) within the watershed. The complexity of the Hg cycle challenges our ability to effectively predict concentrations in upper trophic level fish and wildlife from Hg concentrations in environmental media (soil, water, sediment; Gustin et al. 2016; Sunderland et al. 2016). Identifying appropriate bioindicators based on their relationship with sensitive ecosystems is a critical first step in assessing risk to ecological and human health through long-term Hg monitoring.
Fish species
Fish size class reflects different aspects of trophic transfer of Hg: (1) young fish (<1 year) reflect rapid changes in the availability of MeHg and local conditions; (2) mid-sized fish are important for assessing impacts to fish-eating wildlife such as common loons (Gavia immer), bald eagles (Haliaeettus leucocephalus), osprey (Pandion haliaetus), and North American river otter (Lontra canadensis); while (3) large fish that are at high trophic levels (i.e., game fish) are of particular concern for human health (Driscoll et al. 1994; Millard et al. 2020b).
The impacts on fish health and reproductive welfare are not well established, but have been summarized previously (Depew et al. 2012a, 2012b; Table 4). While fish Hg concentrations are commonly examined for their impacts on humans (i.e., muscle tissue) or for wildlife exposure (i.e., whole body), the MeHg concentrations in fish tissues also impact behavior, reproductive abilities, and overall health of fish. Fish may exhibit impaired reproductive success at relatively low MeHg concentrations as low as 0.04 ppm (ww) (Depew et al. 2012b) and may have adverse visible behavioral impacts at dietary MeHg concentrations of 0.50 ppm, ww or higher (Depew et al. 2012b). Lower reproductive success reduces the size and sustainability of healthy fish populations, which can have adverse impacts on associated populations of piscivores and human recreational and commercial interests. Unlike freshwater fish, there have been few rigorous published studies evaluating toxicity of MeHg to marine fish (Scheuhammer et al. 2015).
Game fish are predatory fish that tend to be at high trophic levels (i.e., trophic levels 4 or 5) and therefore experience several levels of biomagnification of MeHg. Human consumption of game fish in New York State usually includes species such as: walleye from Lake Erie and Lake Ontario; smallmouth and largemouth bass from inland lakes and rivers; and bluefish (Pomatomus saltatrix) and striped bass (Morone saxatilis) from nearshore marine areas, all of which are well reflected in the NYSMD (Fig. 11).
A total of 33,502 fish samples were analyzed for Hg in New York from 1969 to 2017 representing 485 grids for the state (47% represented). Of those grids, 80%, 42%, and 6% had average Hg concentrations above 0.10, above 0.18 and above 0.40, respectively, which are important for the health and reproductive welfare of avian piscivores (Depew et al. 2012a)
There are marked spatial patterns of fish Hg concentrations across New York State (Figs 11, 12). In general fish Hg concentrations are highest in the Adirondack and Catskill regions and the Hudson Highlands of eastern New York State (Millard et al. 2020b). Land cover is an important determinant of this spatial pattern of fish Hg concentrations, with higher concentrations under forest land cover and lower under agricultural land cover (Fig. 13). Forest cover coincides with attributes that facilitate elevated fish Hg concentrations, including abundant wetlands and waters characterized by low nutrients and productivity. Conversely, agricultural lands coincide with lower fish Hg concentrations, partly related to fewer wetlands. Denkenberger et al. (2020) found marked variation in the forms of Hg in rivers draining into Lake Ontario. They observed strong partitioning of inorganic Hg by suspended matter. Watersheds high in agricultural and urban land cover that supplied large quantities of suspended particulate matter tended to largely transport particulate forms of Hg, although this riverine response was muted by the presence of reservoirs. In contrast, in forested landscapes river Hg largely occurred in a dissolved form. Although land cover is an important determinate of fish Hg concentrations for areas with an abundance of forest cover, there is considerable variability in Hg concentrations in game fish (Figs 12, 13).
A total of 25,909 game fish samples (38 species) were analyzed for Hg in New York from 1969 to 2017, representing 452 grid cells with Hg exposure data. Of these grids, 43 percent have average game fish Hg concentrations over 0.22 ppm, with the Adirondack and Catskill Mountain regions and upper Susquehanna River Valley regularly having grids with elevated game fish Hg concentrations
Relationships of game fish Hg concentrations with land cover. The upper panel shows game fish Hg concentrations as a function of forest land cover (Mean Game Fish THg) = 0.117119 + 0.001568 (Mean Percent Forest Cover), F1,483 = 87.54, R2 = 0.1517, p < 0.001) with spatial distribution forest land cover on the right. The lower panel shows game fish Hg concentrations as a function of agricultural land cover (Mean Game Fish THg) = 0.211629 + −0.001341(Mean Percent Agriculture Cover), F1,483 = 18.89, R2 = 0.0354, p < 0.001) with the spatial distribution of agricultural land cover on the right
Avian piscivores
Birds that regularly forage on fish (piscivores) are often at risk from environmental Hg (Table 5). In New York State, larger avian piscivores (e.g., common loon, bald eagle, osprey) tend to have greater Hg body burdens than smaller avian piscivores (e.g., common merganser (Mergus merganser), belted kingfisher (Megaceryle alcyon), roseate tern Sterna dougallii). The greatest risk in New York State among these large piscivores is for common loons in the Adirondack Mountains (Schoch et al. 2020), and for bald eagles foraging in the Catskill Mountains and major rivers (e.g., Saint Lawrence, Hudson, Allegheny, and Susquehanna Rivers; DeSorbo et al. 2020; Fig. 14).
Avian piscivores sampled in New York State from 1970 through 2017. Of the grids sampled for avian piscivores (n = 97), 52 percent of the grids they occupy were above 1.5 ppm of total Hg in their blood (equivalent to a loss of 10% of reproductive success), while 33% were above 2.0 ppm or 20% loss, and 20% over 2.5 ppm or 30% loss (Table 5)
Avian invertivores
Methylmercury from aquatic ecosystems can enter terrestrial food webs via predatory invertebrates, such as dragonflies and spiders (Seewagen et al. 2016; Nelson et al. 2020; Sauer et al. 2020a). Consumers of these and other invertebrates include avian invertivores, which have been identified to be of concern due to MeHg exposure (Evers et al. 2005; Jackson et al. 2011, 2015; Cristol and Evers 2020). The highest Hg concentrations and associated health risk in the state are for the following species: saltmarsh and seaside sparrows (Ammospiza caudacuta and A. maritima, respectively) in the estuaries of Long Island; palm warbler (Setophaga palmarum) and rusty blackbird (Euphagus carolinus) in the Adirondack Mountains; swamp sparrow (Melospiza georgiana) and red-winged blackbird (Agelaius phoeniceus) in the Catskill Mountains; and marsh wren (Cistothorus palustris) in the Montezuma Lakes complex (Fig. 15). Mercury body burdens for these species are at levels that may cause adverse reproductive impacts at a scale of concern.
From 1970 to 2017, a total of 93 grids (representing 9% coverage) have been sampled for avian invertivores. Target songbirds were found in 73 of these grids, and 44% of these birds had >0.4 ppm total blood Hg; 17% were >0.7 ppm; 7% were >1.2 ppm; and 4% were >1.7 ppm (Table 5)
Habitats sensitive to mercury inputs
Habitats where relatively small inputs of Hg can be transported, methylated and bioaccumulate to create areas of concern for fish, wildlife, and people are known as biological Hg hotspots (Evers et al. 2007). Mercury sensitive habitats identified in New York State based on observed Hg concentrations in biota include:
-
1.
Estuaries: Mercury concentrations in blood and feather tissue from the saltmarsh sparrow on Long Island regularly exceeds levels that cause lower reproductive success in songbirds (Lane et al. 2020). Saltmarsh and seaside sparrows are at particular risk because they often feed on spiders—a high MeHg food source (Cristol et al. 2008).
-
2.
Sphagnum bogs and other wetlands: Wetlands are environments with efficient conversion of inorganic Hg to MeHg (Wang et al. 2020). In the Adirondack Park and elsewhere in northern New York State, sphagnum bogs generate elevated levels of MeHg. The transfer of MeHg within and between aquatic and terrestrial food webs are important pathways for biomagnification and create risk for some avian invertivores such as the palm warbler (Setophaga palmarum) and rusty blackbird (Edmonds et al. 2010; Perkins et al. 2020; Sauer et al. 2020a).
-
3.
High-elevation boreal forests: Montane habitats generally receive higher rates of atmospheric Hg deposition due to increased precipitation quantity and cloud cover (Blackwell et al. 2014; Gerson et al. 2017). An elevational gradient of increasing Hg exposure was found in songbirds including Swainson’s (Catharus ustulatus), hermit (C. guttatus), and Bicknell’s (C. bicknelli) thrush on Whiteface Mountain in the Adirondack Park—although the risk to reproductive success is minor (Rimmer et al. 2005, 2020; Sauer et al. 2020b).
-
4.
Streams and rivers in forested watersheds: Based on macroinvertebrates (e.g., odonate larvae) and fish, watersheds with higher forest cover and wetlands have higher MeHg concentrations that frequently exceed fish-related guidelines for human health and wildlife (Riva-Murray et al. 2020a, 2020b; Millard et al. 2020b). The belted kingfisher (Megaceryle alcyon) and Louisiana waterthrush (Parkesia motacilla) are good bioindicators for such systems (Evers et al. 2005; Jackson et al. 2011).
-
5.
Acidic lakes with wetland shorelines in forested watersheds: While most lakes have the potential for high trophic level biota with elevated Hg body burdens, oligotrophic lakes with a pH below 6.3, with significant shoreline wetlands, and/or fluctuating water levels are of greatest concern for piscivorous species such as game fish (Millard et al. 2020b), the common loon (Schoch et al. 2020) and bald eagle (DeSorbo et al. 2020).
The Northeastern Highlands Ecoregion (particularly the Adirondack and Catskill regions) of New York State are particularly sensitive to Hg pollution. The impact of Hg emissions and deposition is exacerbated by watershed, stream, and lake characteristics in areas with abundant forests and wetlands—areas that enhance Hg inputs, transport, methylation, and uptake to elevate concentrations in aquatic food webs resulting in elevated Hg concentrations in game fish and avian piscivores and invertivores. These regions have also been impacted by historical acid deposition.
Long-term changes in mercury in biota
Multiple bioindicators are needed to confidently track changes. For some biota, such as songbirds, long-term trends in Hg exposure can be tracked with museum specimens and compared with recent sampling (Dzielski et al. 2020; Perkins et al. 2020). There is strong evidence that long-term increases in environmental Hg loads (Drevnick et al. 2012) and subsequent effects have increased significantly since the mid-1800s (Perkins et al. 2020), but linking Hg body burdens of biota in New York State with Hg emission patterns remains challenging (Dzielski et al. 2020).
Time series analysis revealed limited significant trends in Hg concentrations in fish or birds for most ecoregions of New York State (Fig. 16). The most consistent significant decreasing trends were evident across multiple fish species in the Eastern Great Lakes Lowlands (Fig. 16). These trends may reflect decreases in releases of Hg to the Great Lakes region coupled with decreases in atmospheric Hg deposition. Ye et al. (2020) found that lands immediately adjacent to the Great Lakes had among the highest atmospheric Hg deposition in New York State (Fig. 6). To a lesser extent decreasing trends were also evident for largemouth bass, white perch and yellow perch in the Northern Highlands. These decreasing trends are tempered by increasing concentrations of Hg in northern pike (Esox lucius; Fig. 16). Trends in fish Hg concentrations that are inconsistent with those in atmospheric Hg deposition are quite common and can result from the influence of many factors including response lags, climate variation, and shifts in food sources (Wang et al. 2019).
Game fish have been monitored for Hg in the Great Lakes adjacent to New York State for almost 50 years (Richter and Skinner 2020; Fig. 16). Over this period, many environmental factors influencing Hg bioavailability have changed, including Hg deposition rates, climate, hydrology and lake stage, surrounding terrestrial habitat, and food webs. Using fillet concentrations, Hg exposure levels were tracked in 16 fish species over a 40-year period to determine annual changes in fish exposure risk and human risk from consumption (Richter and Skinner 2020). The steepest declines in Hg concentrations occurred from the 1970s–80s for yellow perch (n = 999) and largemouth bass (n = 1344). From the 1980s onward, fish Hg concentrations have continued to generally decline. Zhou et al. (2017) also found decreasing Hg trends (in lake trout, Salvelinus namaycush, and walleye) across the Great Lakes for the past four decades, except in the past decade where there were increases for Lake Erie and Lake Ontario.
Common loons have been monitored for Hg exposure in the Adirondack Park since 1998 (Schoch et al. 2020) and optimal sampling strategies are now identified, although lake acidity (Yang et al. 2020) and likely other abiotic factors (Buxton et al. 2020) need to be considered when evaluating trends. Mercury can be elevated in the fish that loons consume, which may adversely affect loon populations. As the top predator in montane lake ecosystems, loons show Hg concentrations that are indicative of risk throughout their breeding range in New York State. Using Hg in blood and egg samples from breeding loons in 116 lakes throughout Adirondack Park from 1998 to 2016, Schoch et al. (2020) tracked MeHg availability and evaluated risk to the common loon (Fig. 16). Concentrations of Hg in breeding loons increased 5.7 percent per year from 1998 to 2010, and then stabilized from 2010 to 2016. Recovery of Hg concentrations in loons has been delayed compared to trends in local atmospheric Hg deposition (Fig. 8), even though there has been a long-term recovery of lakes in the Adirondack region to decreases in acidic deposition (Waller et al. 2012; Driscoll et al. 2016).
Mercury exposure high enough to cause reproductive impacts has been identified in saltmarsh sparrows (Lane et al. 2011, 2020), a species classified as endangered on the International Union for Conservation of Nature (IUCN) Red List. Since 2000, monitoring projects have tracked Hg concentrations in saltmarsh sparrow blood to understand how the risk of Hg effects has changed over time across Long Island (Lane et al. 2020; Fig. 16). Risk of Hg exposure is declining, but remains elevated across New York State marshes, and there is considerable variation in blood Hg concentrations within the breeding season. Concentrations peak in mid-July and such seasonal patterns need to be considered for long-term monitoring. Most marshes have cyclical patterns of annual Hg exposure, which may relate to annual variation in local Hg deposition, tidal marsh flooding, and variation in food availability.
For fish species, more than 50 years of Hg data are available, and are especially robust for yellow perch and largemouth bass, which show statistically significant declines statewide based on the NYSMD and for the Eastern Great Lakes Lowlands, specifically, the slope parameters are significant for yellow perch (β = −0.022 [−0.033, −0.011]) and largemouth bass (β = −0.015 [−0.030, 0.000]). For birds, the temporal span of data varies from six to more than 20 years among species. The red-eyed vireo (Vireo olivaceus) sampled in Long Island’s forests shows an increasing trend (slope parameter β = 0.26, 95% credible interval = [0.03, 0.49]), yet in nearby estuaries, the saltmarsh sparrow shows a declining trend (slope parameter β = −0.055, 95% credible interval = [−0.144, 0.036]). Ultimately, ecoregions may respond differently to changes in environmental Hg loads, as will taxa. Therefore, use of fish and bird bioindicators for multiple ecoregions is essential to understand and track the availability of MeHg across New York State.
Mercury concentrations in biota of New York State have declined or remained constant over the last four decades, concurrent with decreased air emissions from regional and U.S. sources (Figs. 4, 5, 7, 8). After initial declines, however, concentrations of Hg in some fishes and birds from certain locations have increased in recent years, revealing the complexities of trajectories of Hg recovery (Zhou et al. 2017). Because there is not a demonstrated relationship between atmospheric Hg deposition and biotic uptake (Rimmer et al. 2020; Dzielski et al. 2020), Hg monitoring in biota is of ongoing importance.
Long-term influences from climate change
The results of these studies suggest that climate change could potentially affect future Hg deposition. For example, climate predictions for the northeastern United States anticipate increased precipitation amounts and increased temperature, which may in turn drive increases in Hg deposition. This projection underscores the importance of further reduction of anthropogenic Hg emissions in order to address the potential influence climate change may have on Hg exposure.
Factors affecting Hg transport, transformations, and bioaccumulation include: (1) increases in rainfall amount and variability, which create conditions of increased transport and leaching from soil and enhanced wet-dry cycles that augment methylation (Burns et al. 2014a; Watras et al. 2020; Wang et al. 2020); (2) enhanced growth of trees which is a major pathway for atmospheric Hg deposition (Blackwell et al. 2014; Jiskra et al. 2018); (3) higher water temperatures which are associated with higher Hg methylation rates and fish Hg concentrations, and strengthen and extend the duration of thermal stratification in lakes which may increase depletion of dissolved oxygen and methylation of Hg (Bodaly et al. 1993; Warren et al. 2016); and (4) increases in water temperature and fluctuating water tables in stream water enhancing MeHg concentrations (Burns et al. 2014b).
Recent evidence suggests that climate-related factors influence patterns of Hg exposure in aquatic and terrestrial biota. Increasing temperature and rainfall, combined with shifting habitat distributions, will shape future Hg deposition, transport, and methylation, and in turn, exposure risk. Understanding how these large-scale factors will play out on smaller spatial and temporal scales will require continued research and monitoring (Swinton and Nierzwicki-Bauer 2020; Adams et al. 2020).
What are key mercury policy connections in New York State and beyond?
While the timing and magnitude of the response will vary, further controls on Hg emission sources are expected to lower Hg concentrations in food webs, yielding multiple benefits to fish, wildlife, and people in New York State and the surrounding region. Improvements are anticipated to be greatest for inland surface waters and will be roughly proportional to declines in Hg deposition (Knightes et al. 2009), which most closely track trends in regional and U.S. air emissions. This scientific synthesis of Hg in air, water, invertebrates, fish, and birds characterizes the status and effects of Hg pollution across New York State. Information from existing data and Hg monitoring programs can inform many of the regional, national, and global policy initiatives currently implemented or in process.
U.S. mercury emissions
Mercury pollution in the U.S. is regulated by an array of state and federal regulations (see: http://www.epa.gov/hg/). There has been substantial progress in regulatory efforts to decrease Hg emissions from major source categories. Specifically, the USEPA has: (1) finalized maximum standards for Hg from coal-fired power plants; (2) created national emissions standards for hazardous air pollutants for gold ore processing and production facilities; (3) finalized rules to control Hg emissions from Portland cement manufacturing facilities; and (4) proposed new source performance standards and emissions guidelines for new and existing sewage sludge incinerators. Mercury reductions from electric utilities have also occurred indirectly through controls on emissions of sulfur dioxide and nitrogen oxides (Zhang et al. 2016).
The MATS rule has resulted in marked decreases in Hg emissions from electric utilities (Fig. 4). This rule is integral to meeting U.S. commitments under the international Minamata Convention on Mercury. In 2020, the USEPA decided to rescind the finding that regulatory Hg is “appropriate and necessary”, a modification that has led to challenges to the MATS rule and its potential repeal.
Many states have implemented their own regulations to limit Hg emissions, which have often been more stringent than federal regulations (Rallo et al. 2012). One novel approach is use of provisions of the Clean Water Act and establishing a total maximum daily load (TMDL) for Hg that can have an indirect effect on emissions (https://neiwpcc.org/our-programs/nps/mercury/mercury-tmdl/). New York State and the six New England states began implementation of the Northeast Regional Mercury Total Maximum Daily Load in 2007 to reduce fish Hg concentrations in all surface water bodies to less than 0.3 ppm. The TMDL effort has harnessed and focused efforts within the Northeast to measure and identify impaired water bodies and to reduce Hg use and emissions within the region but has encountered some hurdles as well because of limited ability of the states to affect Hg emissions outside their region (Selin 2011).
Minamata Convention on Mercury
The Minamata Convention on Mercury is a global treaty intended to protect human health and the environment from anthropogenic emissions and releases of Hg. In 2013 the text of the Convention was approved by delegates representing nearly 140 countries. The Convention was adopted and signed in 2013 at the Diplomatic Conference in Kumamoto, Japan. The Convention entered into force in 2017. More than 120 countries have ratified or accessioned the Convention, including the United States (see https://www.mercuryconvention.org/). Pursuant to Article 23 of the Minamata Convention, a governing body called the Conference of the Parties (COP) was established. The COP is responsible for advancing implementation of the Convention and for keeping the Convention under continuous review and evaluation. To evaluate the effectiveness of the Minamata Convention’s success, Hg monitoring efforts at the global level are planned (Evers and Sunderland 2019). Decisions relevant to these responsibilities are made during meetings of the COP. The Minamata Convention is expected to provide substantial benefits to the U.S. in excess of $100 billion as cumulative lifetime health benefits as well as economic benefits, primarily due to improved health outcomes from consumption of marine fish (Giang and Selin 2016). Residents of New York are expected to realize substantial benefits from implementation of the Minamata Convention due to anticipated decreases in atmospheric Hg deposition from global sources (Lin et al. 2012), as well because of state’s abundant coastal marine waters and previously identified high levels of marine fish consumption and associated high Hg blood levels in coastal areas (Mahaffey et al. 2009).
Science informs policy—a mercury policy timeline
There has been substantial progress in regulating Hg use, emissions, and releases over the past four decades. The scientific understanding of the harm to human health and the environment from Hg increased in the 1980s and 1990s, when U.S. policies to control Hg trade, its use in products and industry, and its release in waste streams began to be instilled at state and regional levels (e.g., the Great Lakes and New England).
In parallel, local policymaking and national assessment of Hg effects were conducted that would eventually result in the MATS rule. Efforts to monetize the benefits of controls on Hg releases have found that the direct health benefits of controls are sizable (Giang and Selin 2016). The MATS rule was important in establishing a science-based policy for the United States to participate in a leadership role for creating the Minamata Convention on Mercury. Tracking Hg in the environment and its impact on human health is an important next step in the control of Hg use, emissions, and releases. The relationships among key compartments such as air, biota, and humans are complex and often not linear.
The complexity and interactions of the drivers behind the transport of Hg, methylation, and subsequent transfer through the food web requires monitoring to understand and evaluate the success of the MATS rule and the Minamata Convention. Tracking Hg over time also requires carefully designed sampling approaches that can generate standardized and comparable data over time. Preliminary approaches are being generated for the Minamata Convention (e.g., biota; Evers and Sunderland 2019) and with an expectation of initiation in the near future.
References
Adams EM, Sauer AK, Lane O, Regan K, Evers DC (2020) The effects of climate, habitat, and trophic position on methylmercury bioavailability for New York songbirds. Ecotoxicology. https://doi.org/10.1007/s10646-019-02151-w
Adams EM, Gulka JE, Yang Y, Burton MEH, Burns DA, Buxton V, Cleckner L, DeSorbo C, Driscoll CT, Evers DC, Fisher N, Lane O, Mao H, Riva-Murray K, Millard G, Razavi R, Richter W, Sauer AK, Schoch N (2020) Distribution and trends of mercury in aquatic and terrestrial biota of New York, USA: a synthesis of 50 years of research and monitoring. BRI Report No. 2020-24
Åkerblom S, Bishop K, Björn E, Lambertsson L, Eriksson T, Nilsson MB (2013) Significant interaction effects from sulfate deposition and climate on sulfur concentrations constitute major controls on methylmercury production in peatlands. Geochim Cosmochim Acta 102:1–11. https://doi.org/10.1016/j.gca.2012.10.025
Amos HM, Jacob DJ, Streets DG, Sunderland EM (2013) Legacy impacts of all-time anthropogenic emissions on the global mercury cycle. Glob Biogeochem Cycles 27:410–421
Blackwell BD, Driscoll CT, Maxwell JA, Holsen TM (2014) Changing climate alters inputs and pathways of mercury deposition to forested ecosystems. Biogeochemistry 119:215–228
Blackwell BD, Driscoll CT (2015) Deposition of mercury in forests along a montane elevation gradient. Environ Sci Technol 49:5363–5370
Bloomfield JA, Quinn SO, Scrudato RJ, Long D, Richards A, Ryan F (1980) Atmospheric and watershed inputs of mercury to Cranberry Lake, St. Lawrence County, New York. In: Toribara TY, Miller MW, Morrow PE (eds) Polluted rain. Environmental science research. Springer, Boston, MA. https://doi.org/10.1007/978-1-4613-3060-8_9
Bodaly RA, Rudd JWM, Fudge RJP, Kelly CA (1993) Mercury concentrations in fish related to size of remote Canadian shield lakes. Can J Fisheries Aquat Sci 50:980–987
Bourtsalas AT, Themelis NJ (2019) Major sources of mercury emissions to the atmosphere: the US case. Waste Manag 85:90–94
Buck DG, Evers DC, Adams E, DiGangi J, Beeler B, Samánek J, Petrlik J, Turnquist MA, Speranskaya O, Regan K, Johnson S (2019) A global-scale assessment of fish mercury concentrations and the identification of biological hotspots. Sci Total Environ 687:956–966
Burns DA, Riva-Murray K, Bradley PM, Aiken GR, Brigham ME (2012) Landscape controls on total and methyl Hg in the upper Hudson River basin, New York, USA. J Geophys Res Biogeosciences 117. https://doi.org/10.1029/2011JG001812
Burns DA, Woodruff LG, Bradley PM, Cannon WF (2014a) Mercury in the soil of two contrasting watersheds in the Eastern United States. PloS ONE 9:e86855
Burns DA, Nystrom EA, Wolock DM, Bradley PM, Riva-Murray K (2014b) An empirical approach to modeling methylmercury concentrations in an Adirondack stream watershed. J Geophys Res Biogeosciences 119:1970–1984
Buxton VL, Evers DC, Schoch N (2020) The influence of biotic and abiotic factors on banded common loon (Gavia immer) reproductive success in a remote, mountainous region of the northeastern United States. Ecotoxicology
Cain A, Morgan JT, Brooks N (2011) Mercury policy in the Great Lakes states: past successes and future opportunities. Ecotoxicology 20:1500–1511
Carpenter B, Gelman A, Hoffman MD, Lee D, Goodrich B, Betancourt M, Brubaker M, Guo J, Li P, Riddel A (2017) Stan: a probabilistic programming language. J Stat Softw 76. https://doi.org/10.18637/jss.v076.i01
Chen CYKB, Shaw AL, Curtis A, Taylor MS, Montedesca MR, Driscoll CT (2020) The influence of nutrient loading on methylmercury availability in long island estuaries. Environ Pollut, (in press). https://doi.org/10.1016/j.envpol.2020.11510
Choi H-D, Holsen TM, Hopke PK (2008) Atmospheric mercury (Hg) in the Adirondacks: concentrations and sources. Environ Sci Technol 42:5644–5653
Coleman Wasik JK, Engstrom DR, Mitchell CPJ, Swain EB, Monson BA, Balogh SJ, Jeremiason JD, Branfireun BA, Kolka RK, Almendinger. JE (2015) The effects of hydrologic fluctuation and sulfate regeneration on mercury cycling in an experimental peatland. J Geophys Res Biogeosciences 120:1697–1715
Cristol DA, Brasso RL, Condon AM, Fovargue RE, Friedman SL, Hallinger KK, Monroe AP, White AE (2008) The movement of aquatic mercury through terrestrial food webs. Science 320:335–335
Cristol DA, Evers DC (2020) The impact of mercury on North American songbirds: effects, trends, and predictive factors. Ecotoxicology 29:1107–1116
Denkenberger JS, Driscoll CT, Branfireun BA, Eckley CS, Cohen M, Selvendiran P (2012) A synthesis of rates and controls on elemental mercury evasion in the Great Lakes Basin. Environ Pollut 161:291–298
Denkenberger J, Fakhraei H, Branfireun B, Mason E, Driscoll CT (2020) Watershed influences on mercury in tributaries to lake Ontario. Ecotoxicology. https://doi.org/10.1007/s10646-019-02157-4
Depew DC, Basu N, Burgess NM, Campbell LM, Evers DC, Grasman KA, Scheuhammer AM (2012a) Derivation of screening benchmarks for dietary methylmercury exposure for the common loon (Gavia immer): rationale for use in ecological risk assessment. Environ Toxicol Chem 31:2399–2407
Depew DC, Basu N, Burgess NM, Campbell LM, Devlin EW, Drevnick PE, Hammerschmidt CR, Murphy CA, Sandheinrich MB, Wiener JG (2012b) Toxicity of dietary methylmercury to fish: derivation of ecologically meaningful threshold concentrations. Environ Toxicol Chem 31:1536–1547
DeSorbo CR, Burgess NM, Nye PE, Loukmas JJ, Brant HA, Burton MEH, Persico CP, Evers DC (2020) Bald eagle mercury exposure varies with region and site elevation in New York, USA. https://doi.org/10.1007/s10646-019-02153-8
Dittman JA, Shanley JB, Driscoll CT, Aiken GR, Chalmers AT, Towse JE, Selvendiran P (2010) Mercury dynamics in relation to dissolved organic carbon concentration and quality during high flow events in three northeastern U.S. streams. Water Resour Res 46:W07522. https://doi.org/10.1029/2009WR008351
Drevnick PE, Engstrom DR, Driscoll CT, Balogh SJ, Kamman NC, Long DT, Muir DGC, Parsons MJ, Rolfhus KR, Rossmann R, Swain EB (2012) Spatial and temporal patterns of mercury accumulation in lacustrine sediments across the Laurentian Great Lakes region. Environ Pollut 161:252–260
Driscoll CT, Yan C, Schofield CL, Munson R, Holsapple J (1994) The mercury cycle and fish in the Adirondack lakes. Environ Sci Technol 28:136A–143A
Driscoll CT, Han Y-J, Chen CY, Evers DC, Lambert KF, Holsen TM, Kamman NC, Munson RK (2007) Mercury contamination in forest and freshwater ecosystems in the Northeastern United States. BioScience 57:17–28
Driscoll CT, Chen CY, Hammerschmidt CH, Mason RP, Gilmour CC, Sunderland EM, Greenfield B, Lamborg CH (2012) Nutrient supply and mercury dynamics in marine ecosystems: a conceptual model. Environ Res 119:118–131
Driscoll CT, Mason RP, Chan HM, Jacob DJ, Pirrone N (2013) Mercury as a global pollutant: Sources, pathways and effects. Environ Sci Technol 47:4967–4983
Driscoll CT, Driscoll KM, Fakhraei H, Civerolo K (2016) Long-term temporal trends and spatial patterns in the acid-base chemistry of lakes in the Adirondack region of New York in response to decreases in acidic deposition. Atmos Environ 146:5–14. https://doi.org/10.1016/j.atmosenv.2016.08.034
Dzielski SA, Razavi NR, Twining CW, Cleckner LB, Rohwer VG (2020) Reconstructing avian mercury concentrations through time using museum specimens from New York State. Ecotoxicology. https://doi.org/10.1007/s10646-019-02123-0
Eagles-Smith CA, Ackerman JT, De La Cruz SE, Takekawa JY (2009) Mercury bioaccumulation and risk to three waterbird foraging guilds is influenced by foraging ecology and breeding stage. Environ Pollut 157:1993–2002
Eagles-Smith CA, Ackerman JT, Willacker JJ, Tate MT, Lutz MA, Fleck JA, Stewart AR, Wiener JG, Evers DC, Lepak JM, Davis JA, Pritz CF (2016) Spatial and temporal patterns of mercury concentrations in freshwater fish across the Western United States and Canada. Sci Total Environ 568:1171–1184
Edmonds ST, Evers DC, Cristol DA, Mettke-Hofmann C, Powell LL, McGann AJ, Armiger JW, Lane OP, Tessler DF, Newell P, Heyden K (2010) Geographic and seasonal variation in mercury exposure of the declining rusty blackbird. Condor 112:789–799
Evers DC (2018) The effects of methylmercury on wildlife: a comprehensive review and approach for interpretation. Encyclopedia Anthropocene 5:181–194
Evers DC, Clair TA (2005) Mercury in northeastern North America: a synthesis of existing databases. Ecotoxicology 14:7–14
Evers DC, Sunderland E (2019) Technical information report on mercury monitoring in biota. United Nations Environment Programme, Geneva, Switzerland
Evers DC, Burgess NM, Champoux L, Hoskins B, Major A, Goodale WM, Taylor RJ, Poppenga R, Daigle T (2005) Patterns and interpretation of mercury exposure in freshwater avian communities in northeastern North America. Ecotoxicology 14:193–221
Evers DC, Han YJ, Driscoll CT, Kamman NC, Goodale MW, Lambert KF, Holsen TM, Chen CY, Clair TA, Butler T (2007) Biological mercury hotspots in the northeastern United States and southeastern Canada. BioScience 57:29–43
Evers DC, Wiener JG, Basu N, Bodaly RA, Morrison HA, Williams KA (2011) Mercury in the Great Lakes region: bioaccumulation, spatiotemporal patterns, ecological risks, and policy. Ecotoxicology 20:1487–1499
Evers DC, Keane SE, Basu N, Buck D (2016) Evaluating the effectiveness of the Minamata Convention on Mercury: Principles and recommendations for next steps. Sci Total Environ 569-570:888–903
Gerson JR, Driscoll CT, Demers J, Sauer A, Blackwell B, Monstesdeoca M, Shanley J, Ross D (2017) Deposition of mercury in forests across a montane elevation gradient: elevational and seasonal patterns in methylmercury inputs and production. J Geophys Res Biogeosciences 122:1922–1939
Giang A, Selin NE (2016) Benefits of mercury controls for the United States. Proc Natl Acad Sci USA 113:286–291. https://doi.org/10.1073/pnas.1514395113
Gilmour CC, Henry EA (1991) Mercury methylation in aquatic systems affected by acid deposition. Environ Pollut 71:131–169
Gilmour CC, Podar M, Bullock AL, Graham AM, Brown SD, Somenahally AC, Johs A, Hurt RA, Bailey KL, Elias DA (2013a) Mercury methylation by novel microorganisms from new environments. Environ Sci Technol 47:11810–11820
Gilmour CC, Podar M, Bullock AL, Graham AM, Brown SD, Somenahally AC, Johs A, Hurt Jr RA, Bailey KL, Elias DA (2013b) Mercury methylation by novel microorganisms from new environments. Environ Sci Technol 47:11810–11820
Glass GE, Sorensen JA, Schmidt KW, Rapp Jr GR (1990) New source identification of mercury contamination in the Great Lakes. Environ Sci Technol 24:1059–1069
GLRC (Great Lakes Regional Collaboration) (2010) Great Lakes mercury emission reduction strategy. 30 June 2011. http://www.glrc.us/initiatives/toxics/index.html
Great Lakes Fish Advisory Workgroup (2007) A protocol for mercury based fish consumption advice: an addendum to the 1993 protocol for a uniform Great Lakes sport fish consumption advisory. Minnesota Department of Health, pp 30. https://www.health.state.mn.us/communities/environment/fish/docs/consortium/mercuryprot.pdf
Goodrich B, GabryJ, Ali I, Brilleman S (2018) Bayesian applied regression modeling via Stan [WWW Document]. http://mc-stan.org
Grieb TM, Fisher NS, Karimi R, Levin L (2020) An assessment of temporal trends in mercury concentrations in fish. Ecotoxicology. https://doi.org/10.1007/s10646-019-02112-3
Gustin MS, Evers DC, Bank MS, Hammerschmidt CR, Pierce A, Basu N, Blum J, Bustamante P, Chen C, Driscoll CT, Horvat M, Jaffe D, Pacyna J, Pirrone N, Selin N (2016) Importance of integration and implementation of emerging and future mercury research into the Minamata Convention. Environ Sci Technol 50:2767–2770
Hintelmann H, Harris R, Heyes A, Hurley JP, Kelly CA, Krabbenhoft DP, Lindberg S, Rudd JW, Scott KJ, St. Louis VL (2002) Reactivity and mobility of new and old mercury deposition in a boreal forest ecosystem during the first year of the METAALICUS study. EnvironSci Technol 36:5034–5040
Hsu-Kim H, Kucharzyk KH, Zhang T, Deshusses MA (2013) Mechanisms regulating mercury bioavailability for methylating microorganisms in the aquatic environment: a critical review. Environ Sci Technol 47:2441–2456
Hsu-Kim H, Eckley CS, Achá D, Feng X, Gilmour CC, Jonsson S, Mitchell CP (2018) Challenges and opportunities for managing aquatic mercury pollution in altered landscapes. Ambio 47:141–169
Jackson AK, Evers DC, Etterson MA, Condon AM, Folsom SB, Detweiler J, Schmerfeld J, Cristol DA (2011) Mercury exposure affects the reproductive success of a free-living terrestrial songbird, the Carolina Wren (Thryothorus ludovicianus). Auk 128:759–769
Jackson AK, Evers DC, Adams EM, Cristol DA, Eagles-Smith C, Edmonds ST, Gray CE, Hoskins B, Lane OP, Sauer A, Tear T (2015) Songbirds as sentinels of mercury in terrestrial habitats of eastern North America. Ecotoxicology. 24:453–467
Jiskra M, Sonke JE, Obrist D, Bieser J, Ebinghaus R, Myhre CL, Pfaffhuber KA, Wängberg I, Kyllönen K, Worthy D, Martin LG (2018) A vegetation control on seasonal variations in global atmospheric mercury concentrations. Nat Geoscience 11:244–250
Karimi R, Fitzgerald TP, Fisher NS (2012) A quantitative synthesis of mercury in commercial seafood and implications for exposure in the United States. Environ Health Perspect 120:1512–1519
Karimi R, Frisk M, Fisher NS (2013) Contrasting food web factor and body size relationships with Hg and Se concentrations in marine biota. PloS ONE 8(9):e74695
Knightes CD, Sunderland EM, Barber MC, Johnson JM, Ambrose RB (2009) Application of ecosystem-scale fate and bioaccumulation models to predict fish mercury response times to changes in atmospheric deposition. Environ Toxicol Chemis 28:881–893
Kocman D, Wilson S, Amos H, Telmer K, Steenhuisen F, Sunderland E, Mason RP, Outridge P, Horvat M (2017) Toward an assessment of the global inventory of present day mercury releases to freshwater environments. Int J Environ Res Public Health 14:138. https://doi.org/10.3390/ijerph14020138
Lane OP, O’Brien KM, Evers DC, Hodgman TP, Major A, Pau N, Ducey MJ, Taylor R, Perry D (2011) Mercury in breeding saltmarsh sparrows (Ammodramus caudacutus caudacutus). Ecotoxicology 20:1984–1991
Lane O, Adams EM, Pau N, O’Brien KM, Regan R, Farina M, Schneider Moran T, Zarudsky J (2020) Long-term monitoring of mercury in adult saltmarsh sparrows breeding in Maine, Massachusetts and New York, USA 2000-2017. Ecotoxicology 29:1148–1160
Latifovic R, Pouliot D, Olthof I (2017) Circa 2010 land cover of Canada: local optimization methodology and product development. Remote Sens 9(11):1098
Lee CS, Lutcavage ME, Chandler E, Madigan DJ, Cerrato RM, Fisher NS (2016) Declining mercury concentrations in bluefin tuna reflect reduced emissions to the North Atlantic Ocean. Environ Sci Technol 50:12825–12830
Lin CJ, Shetty SK, Pan L, Pongprueksa P, Jang C, Chu HW (2012) Source attribution for mercury deposition in the contiguous United States: Regional difference and seasonal variation. J Air Waste Manag Assoc 62:52–63
Mahaffey KR, Clickner RP, Jeffries RA (2009) Adult women’s blood mercury concentrations vary regionally in the United States: association with patterns of fish consumption (NHANES 1999–2004). Environ Health Perspect 117:47–53. https://doi.org/10.1289/ehp.11674.
Millard GD, Riva-Murray K, Burns DA, Montesdeoca MR, Driscoll CT (2020a) The impact of lime additions on mercury dynamics in stream chemistry and macroinvertebrates: a comparison of watershed and direct stream addition management strategies. Ecotoxicology. https://doi.org/10.1007/s10646-020-02224-1
Millard GD, Driscoll CT, Montesdeoca M, Yang Y, Taylor M, Boucher S, Shaw A, Richter W, Paul E, Parker C, Yokota K (2020b) Patterns and trends of fish mercury in New York State. Ecotoxicology. https://doi.org/10.1007/s10646-020-02163-x
Milford JA, Pienciak A (2009) After the clean air mercury rule: prospects for reducing mercury emissions from coal-fired power plants. Environ Sci Technol 43:2669–2673. https://doi.org/10.1021/es802649u
NEI (National Emissions Inventory) for Hazardous Air Pollutants (2014) U.S. Environmental Protection Agency. https://www.epa.gov/airemissions-inventories/national-emissions-inventorynei
Nelson SJ, Chen CY, Kahl JS (2020) Dragonfly larvae as biosentinels of Hg bioaccumulation in Northeastern and Adirondack lakes: relationships to abiotic factors. Ecotoxicology. https://doi.org/10.1007/s10646-019-02149-4
Obrist D, Kirk JL, Zhang L, Sunderland EM, Jiskra M, Selin NE (2018) A review of global environmental mercury processes in response to human and natural perturbations: changes of emissions, climate, and land use. Ambio 47:116–140
Olson CI, Fahraei H, Driscoll CT (2020) Mercury emissions, atmospheric concentrations, and wet deposition across conterminous United States: changes over 20 years of monitoring. Environ Sci Technol Lett 7:376–381
Perkins M, Lane OP, Evers DC, Sauer A, Adams EM, O’Driscoll NJ, Edmonds ST, Jackson AK, Hagelin JC, Trimble J, Sunderland EM (2020) Historical patterns in mercury exposure for North American songbirds. Ecotoxicology 29:1161–1173
Podar M, Gilmour CC, Brandt CC, Soren A, Brown SD, Crable BR, Palumbo AV, Somenahally AC, Elias DA (2015) Global prevalence and distribution of genes and microorganisms involved in mercury methylation. Sci Adv 1(9):e1500675. https://doi.org/10.1126/sciadv.1500675
R Core Team (2018) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-9000-07-0 [WWW Document]. http://www.r-project.org/
Rallo M, Lopez-Anton MA, Contreras ML, Maroto-Valer MM (2012) Mercury policy and regulations for coal-fired power plants. Environ Sci Pollut Res 19:1084–1096
Razavi R, Halfman JD, Cushman SF, Massey T, Beutner R, Foust J, Gilman B, Cleckner L (2020) Mercury concentrations in fish and invertebrates of the Finger Lakes in central New York. Ecotoxicology. https://doi.org/10.1007/s10646-019-02132-z
Richter W, Skinner L (2020) Mercury in the fish of New York’s Great Lakes: a quarter century of near stability. Ecotoxicology. https://doi.org/10.1007/s10646-019-02130-1
Rimmer CC, McFarland KP, Evers DC, Miller EK, Aubry Y, Busby D, Taylor RJ (2005) Mercury concentrations in Bicknell’s thrush and other insectivorous passerines in montane forests of northeastern North America. Ecotoxicology 14:223–240
Rimmer CC, Lloyd JD, McFarland KP, Evers DC, Lane OP (2020) Patterns of blood mercury variation in two long-distance migratory thrushes on Mount Mansfield, Vermont. Ecotoxicology 29:1174–1182
Riva-Murray K, Razavi R, Richter W, Cleckner L, Burns D (2020a) Mercury in fish from New York’s streams and rivers: recent spatial patterns and long-term trends. Ecotoxicology. https://doi.org/10.1007/s10646-020-02225-0
Riva-Murray K, Bradley PM, Brigham ME (2020b) Methylmercury—total mercury ratios in predator and primary consumer macroinvertebrates of Adirondack streams (New York, USA). Ecotoxicology. https://doi.org/10.1007/s10646-020-02191-7
Sauer AK, Driscoll CT, Evers DC, Adams EM, Yang Y (2020a) Mercury exposure in songbird communities in Sphagnum bog and upland forest ecosystems in the Adirondack Park (New York, USA). Ecotoxicology. https://doi.org/10.1007/s10646-019-02142-x
Sauer AK, Driscoll CT, Evers DC, Adams EM, Yang Y (2020b) Mercury exposure in songbird communities along an elevational gradient on Whiteface Mountain, Adirondack Park (New York, USA). Ecotoxicology. https://doi.org/10.1007/s10646-020-02175-7
Scheuhammer A, Braune B, Chan HM, Frouin H, Krey A, Letcher R, Loseto L, Noël M, Ostertag S, Ross P, Wayland M (2015) Recent progress on our understanding of the biological effects of mercury in fish and wildlife in the Canadian Arctic. Sci Total Environ 509:91–103
Schmeltz D, Evers DC, Driscoll CT, Artz R, Cohen M, Gay D, Haeuber R, Krabbenhoft DP, Mason R, Morris K, Wiener JG (2011) MercNet: a national monitoring network to assess responses to changing mercury emissions in the United States. Ecotoxicology 20:1713–1725
Schoch N, Yang Y, Yanai R D, Buxton VL, Evers DC, Driscoll CT (2020) Spatial patterns and temporal trends in mercury concentrations in common loons (Gavia immer) from 1998 to 2016 in New York’s Adirondack Park: has this top predator benefitted from mercury emission controls? Ecotoxicology. https://doi.org/10.1007/s10646-019-02119-w
Seewagen CL, Cristol DA, Gerson AR (2016) Mobilization of mercury from lean tissues during simulated migratory fasting in a model songbird. Sci Rep 6:25762
Selin NE (2011) Science and strategies to reduce mercury risks: a critical review. J Environ Monit 13:2389–2399. https://doi.org/10.1039/c1em10448a
Stan Development Team (2018) Rstan: the R interface to Stan [WWW Document]. http://mc-stan.org
Streets DG, Horowitz HM, Jacob DJ, Lu Z, Levin L, Ter Schure AFH, Sunderland EM (2017) Total mercury released to the environment by human activities. Environ Sci Technol 51:5969–5977
Streets DG, Horowitz HM, Lu Z, Levin L, Thackray CP, Sunderland EM (2019) Global and regional trends in mercury emissions and concentrations, 2010–2015. Atmos Environ 201:417–427
Sunderland EM, Driscoll Jr CT, Hammitt JK, Grandjean P, Evans JS, Blum JD, Chen CY, Evers DC, Jaffe DA, Mason RP, Goho S, Jacobs W (2016) Benefits of regulating hazardous air pollutants from coal and oil-fired utilities in the United States. Environ Sci Technol 50:2117–2120
Sunderland EM, Li M, Bullard K (2018) Decadal changes in the edible supply of seafood and methylmercury exposure in the United States. Environ Health Perspect 126:017006
Swinton MW, Nierzwicki-Bauer SA (2020) Mercury increase in Lake Champlain fish: links to fishery dynamics and extreme climatic events. Ecotoxicology. https://doi.org/10.1007/s10646-019-02148-5
Taylor MS, Driscoll CT, Lepak JM, Josephson DC, Jirka KJ, Kraft CE (2020) Temporal trends in fish mercury concentrations in an Adirondack Lake managed with a continual predator removal program. Ecotoxicology. https://doi.org/10.1007/s10646-019-02156-5
Todorova S, Driscoll CT, Effler SW, O’Donnell S, Matthews DA, Todorov DL, Gindlesperger S (2014) Changes in the long-term supply of mercury species to the upper mixed waters of a recovering lake. Environ Pollut 185:314–321
UN Environment (2019) Global mercury assessment 2018. UN Environment Programme, Chemicals and Health Branch Geneva, Switzerland
U.S. EPA (Environmental Protection Agency) (1997) Great Lakes binational toxics strategy. https://archive.epa.gov/greatlakes/p2/web/pdf/bnssign.pdf
US Environmental Protection Agency (2020) National Emissions Inventory (NEI). Available at https://www.epa.gov/air-emissions-inventories/nationalemissions-inventory-nei
USEPA (Environmental Protection Agency) (2001) Water quality criterion for the protection of human health: methylmercury. EPA-823-R-01-001
United States Food and Drug Administration and United States Environmental Protection Agency (FDA/EPA) (2019) Technical Information on Development of FDA/EPA Advice about Eating Fish for Women Who Are or Might Become Pregnant, Breastfeeding Mothers, and Young Children. https://www.fda.gov/food/metals-and-your-food/technical-information-development-fdaepa-advice-about-eating-fish-women-who-are-or-might-become
Waller K, Driscoll C, Lynch J, Newcomb D, Roy K (2012) Long-term recovery of lakes in the Adirondack region of New York to decreases in acidic deposition. Atmos Environ 46:56–64
Warren DR, Kraft CE, Josephson DC, Driscoll CT (2016) Acid rain recovery may help to mitigate the impacts of climate change on thermally sensitive fish in lakes across eastern North America. Glob Change Biol 23(6):2149–2153. https://doi.org/10.1111/gcb.13568
Wang F, Outridge PM, Feng X, Meng B, Heimbürger-Boavida LE, Mason RP (2019) How closely do mercury trends in fish and other aquatic wildlife track those in the atmosphere?–Implications for evaluating the effectiveness of the Minamata Convention. Sci Total Environ 674:58–70. https://doi.org/10.1016/j.scitotenv.2019.04.101
Wang T, Driscoll CT, Hwang K, Chandler D, Montesdeoca M (2020) Seasonal patterns of total and methyl mercury concentrations in ground and surface waters in natural and restored freshwater wetlands in Northern New York. Ecotoxicology
Warren DR, Kraft CE, Josephson DC, Driscoll CT (2016) Acid rain recovery may help to mitigate the impacts of climate change on thermally sensitive fish in lakes across eastern North America. Glob Change Biol 23(6):2149–2153. https://doi.org/10.1111/gcb.13568
Watras CJ, Teng HY, Latzka AW, Meyer MW, Zhang Z (2020) Near-decadal oscillation of water levels and mercury bioaccumulation in the Laurentian Great Lakes region. Environ Sci Technol Lett 7:89–94
Willacker JJ, Eagles-Smith CA, Kowalski BM, Danehy RJ, Jackson AK, Adams EM, Evers DC, Eckley CS, Tate MT, Krabbenhoft DP (2019) Timber harvest alters mercury bioaccumulation and food web structure in headwater streams. Environ Pollut 253:636–645
Wyn B, Kidd KA, Burgess NM, Curry RA (2009) Mercury biomagnification in the food webs of acidic lakes in Kejimkujik National Park and National Historic Site, Nova Scotia. Can J Fisheries Aquat Sci 66:1532–1545
Yang L, Jin S, Danielson P, Homer C, Gass L, Case A, Costello C, Dewitz J, Fry J, Funk M, Grannemann B, Rigge M, Xian G (2018) A new generation of the United States National Land Cover Database: requirements, research priorities, design, and implementation strategies. ISPRS J Photogramm Remote Sens 146:108–123
Yang Y, Yanai RD, Schoch S, Buxton VL, Gonzalez K, Evers DC, Lampman G (2020) Determining optimal sampling strategies for monitoring mercury and reproductive success in common loons in the Adirondacks of New York. Ecotoxicology. https://doi.org/10.1007/s10646-019-02122-1
Ye Z, Mao H, Driscoll CT (2020) A modeling study on impacts of meteorological variation and anthropogenic emission reductions on atmospheric mercury input to upstate New York ecosystems. Ecotoxicology. https://doi.org/10.1007/s10646-019-02113-2
Ye Z, Mao H, Driscoll CT (2019) Primary effects of changes in meteorology vs. anthropogenic emissions on mercury wet deposition: a modeling study. Atmos Environ 198:215–225
Yu X, Driscoll CT, Montesdeoca M, Evers DC, Duron M, Williams K, Schoch N, Kamman NC (2011) Spatial patterns of mercury in biota of Adirondack, New York lakes. Ecotoxicology 20:1543–1554
Zhang Y, Jacob DJ, Horowitz HM, Chen L, Amos HM, Krabbenhoft DP, Slemr F, Louis VLS, Sunderland EM (2016) Observed decrease in atmospheric mercury explained by global decline in anthropogenic emissions. Proc Natl Acad Sci USA 113:526–531.
Zhou H, Zhou C, Lynam MM, Dvonch JT, Barres JA, Hopke PK, Cohen M, Holsen TM (2017) Atmospheric mercury temporal trends in the northeastern United States from 1992 to 2014: are measured concentrations responding to decreasing regional emissions? Environ Sci Technol Lett 4:91–97. https://doi.org/10.1021/acs.estlett.6b00452
Zhou C, Cohen MD, Crimmins BA, Zhou H, Johnson TA, Hopke PK, Holsen TM (2017) Mercury temporal trends in top predator fish of the Laurentian Great Lakes from 2004 to 2015: are concentrations still decreasing? Environ Sci Technol 51:7386–7394
Acknowledgements
This paper was supported by the New York State Energy Research and Development Authority (NYSERDA) Agreement Number 124842. We would like to thank Greg Lampman for coordinating New York State Hg research over the many years, including the following NYSERDA studies that are also supported in this special issue and featured in this paper (NYSERDA Agreement Numbers: 9956, 16296, 33075, 33075A, 34357, 34358, 37346, 40515, 50462, 50778, 108229, and 113882). To best meet “Fair Principle” objectives, all data associated with this special issue can be accessed under the project “NYSERDA Synthesis of Environmental Mercury Loads in New York State (1969–2017)” at: https://data.ny.gov/Energy-Environment/NYSERDA-Synthesis-of-Environmental-Mercury-Loads-i/v475-sje9. Data were collected by innumerable biologists over the past 50 years and without their work this manuscript would not exist. Any use of trade, product, or firm names is for descriptive purposes only and does not imply endorsement by the U.S. Government. The assistance of Connor Olson in the processing of information on emissions and air concentrations and wet deposition of Hg is greatly appreciated.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Evers, D.C., Sauer, A.K., Burns, D.A. et al. A synthesis of patterns of environmental mercury inputs, exposure and effects in New York State. Ecotoxicology 29, 1565–1589 (2020). https://doi.org/10.1007/s10646-020-02291-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-020-02291-4