Abstract
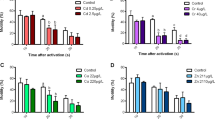
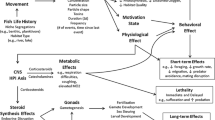
Wastewater effluents are teeming with organisms, nutrients and chemical substances which water treatment processes fail to remove. Among these substances, pharmaceuticals such as antidepressants are a frequent occurrence, and have been reported to lead to severe effects in the physiology and behaviour of non-target marine species across taxa. Venlafaxine (VFX) is one of the most consistently prescribed substances for the treatment of human depressive disorders, acting as a serotonin and norepinephrine reuptake inhibitor. In the present study, the potential effects of this antidepressant on the survival and key behaviours (i.e. movement, aggression and foraging) of white seabream (Diplodus sargus) larvae were addressed. Larvae were submitted to an acute exposure of two different VFX treatments (low concentration, 10 µg L−1; and high concentration, 100 µg L−1) for a total of 48 h. Sampling took place after 24 and 48 h of exposure. Overall, results showed a significant effect of a two-day exposure to VFX in larvae of D. sargus. Survival was significantly reduced by exposure to a high concentration, but behavioural effects of antidepressant exposure were subtle: i.e. increased attack frequency and temporary modulation of capture success. Further research efforts should be directed towards evaluating the potential chronic effects of antidepressants in marine species, if we are to anticipate possible pressures on natural populations, and effectively advice policymakers towards the investment in new and more efficient methods of wastewater treatments.





Similar content being viewed by others
References
Altmann J (1974) Observational study of behavior: sampling methods. Behaviour 49:227–266. https://doi.org/10.1163/156853974X00534
Álvarez-Muñoz D, Rodríguez-Mozaz S, Maulvault AL, Tediosi A, Fernández-Tejedor M, Van den Heuvel F, Kotterman M, Marques A, Barceló D (2015) Occurrence of pharmaceuticals and endocrine disrupting compounds in macroalgaes, bivalves, and fish from coastal areas in Europe. Environ Res 143:56–64. https://doi.org/10.1016/j.envres.2015.09.018
Bates D, Mächler M, Bolker B, Walker S (2014) Fitting linear mixed-effects models using lme4. J Stat Softw. https://doi.org/10.18637/jss.v067.i01
Bisesi JH, Bridges W, Klaine SJ (2014) Reprint of: effects of the antidepressant venlafaxine on fish brain serotonin and predation behavior. Aquat Toxicol 151:88–96. https://doi.org/10.1016/j.aquatox.2014.02.015
Bisesi JH, Sweet LE, van den Hurk P, Klaine SJ (2016) Effects of an antidepressant mixture on the brain serotonin and predation behavior of hybrid striped bass. Environ Toxicol Chem 35:938–945. https://doi.org/10.1002/etc.3114
Brodin T, Fick J, Jonsson M, Klaminder J (2013) Dilute concentrations of a psychiatric drug alter behavior of fish from natural populations. Science. https://doi.org/10.1126/science.1226850
Cascade E, Kalali A, Thase M (2007) Use of antidepressants: expansion beyond depression and anxiety. Psychiatry 4:25–28
Dzieweczynski TL, Hebert OL (2012) Fluoxetine alters behavioral consistency of aggression and courtship in male Siamese fighting fish, Betta splendens. Physiol Behav. https://doi.org/10.1016/j.physbeh.2012.06.007
Fabbri E, Franzellitti S (2016) Human pharmaceuticals in the marine environment: focus on exposure and biological effects in animal species. Environ Toxicol Chem. https://doi.org/10.1002/etc.3131
Galus M, Kirischian N, Higgins S, Purdy J, Chow J, Rangaranjan S, Li H, Metcalfe C, Wilson JY (2013) Chronic, low concentration exposure to pharmaceuticals impacts multiple organ systems in zebrafish. Aquat Toxicol. https://doi.org/10.1016/j.aquatox.2012.12.021
Gaw S, Thomas KV, Hutchinson TH, (2014) Sources, impacts and trends of pharmaceuticals in the marine and coastal environment. Philos Trans R Soc Lond B Biol Sci. https://doi.org/10.1098/rstb.2013.0572
Gaworecki KM, Klaine SJ (2008) Behavioral and biochemical responses of hybrid striped bass during and after fluoxetine exposure. Aquat Toxicol 88:207–213. https://doi.org/10.1016/j.aquatox.2008.04.011
Haedrich RL (2004) Fishes of the North-Eastern Atlantic and Mediterranean. Volume 1. P. J. P. Whitehead, M.-L. Bauchot, J.-C. Hureau, J. Nielsen, E. Tortonese. Q. Rev Biol 61:290–291. https://doi.org/10.1086/415000
Hedgespeth ML, Nilsson PA, Berglund O (2014) Ecological implications of altered fish foraging after exposure to an antidepressant pharmaceutical. Aquat Toxicol. https://doi.org/10.1016/j.aquatox.2013.12.011
Huerta B, Barceló D (2012) Pharmaceuticals in biota in the aquatic environment: analytical methods and environmental implications. Anal Bioanal Chem 404:2611–2624. https://doi.org/10.1007/s00216-012-6144-y
Klaminder J, Hellström G, Fahlman J, Jonsson M, Fick J, Lagesson A, Bergman E, Brodin T (2016) Drug-induced behavioral changes: using laboratory observations to predict field observations. Front Environ Sci 4:81. https://doi.org/10.3389/fenvs.2016.00081
Kohlert JG, Mangan BP, Kodra C, Drako L, Long E, Simpson H (2012) Decreased aggressive and locomotor behaviors in Betta splendens after exposure to fluoxetine. Psychol Rep. https://doi.org/10.2466/02.13.PR0.110.1.51-62
Kreke N, Dietrich, DR (2008) Physiological endpoints for potential SSRI interactions in fish. Crit Rev Toxicol. https://doi.org/10.1080/10408440801891057
Kümmerer K (2009). The presence of pharmaceuticals in the environment due to human use—present knowledge and future challenges. J Environ Manage. https://doi.org/10.1016/j.jenvman.2009.01.023
Little E, Brewer S (2001) Neurobehavioral toxicity in fish. In: Schlenk D, Benson W (eds) Target organ toxicity in marine and freshwater teleosts, vol 2. Taylor & Francisc, London, pp 139–174
Maulvault AL, Santos LHMLM, Camacho C, Anacleto P, Barbosa V, Alves R, Pousão Ferreira P, Serra-Compte A, Barceló D, Rodriguez-Mozaz S, Rosa R, Diniz M, Marques A (2018) Antidepressants in a changing ocean: Venlafaxine uptake and elimination in juvenile fish (Argyrosomus regius) exposed to warming and acidification conditions. Chemosphere. https://doi.org/10.1016/j.chemosphere.2018.06.004
McDonald MD, Gonzalez A, Sloman KA (2011) Higher levels of aggression are observed in socially dominant toadfish treated with the selective serotonin reuptake inhibitor, fluoxetine. Comp Biochem Physiol C Toxicol Pharmacol. https://doi.org/10.1016/j.cbpc.2010.09.006
Melvin, SD (2017) Effect of antidepressants on circadian rhythms in fish: insights and implications regarding the design of behavioural toxicity tests. Aquat Toxicol. https://doi.org/10.1016/j.aquatox.2016.11.007
Øverli Ø, Harris CA, Winberg S (2000) Short-term effects of fights for social dominance and the establishment of dominant-subordinate relationships on brain monoamines and cortisol in rainbow trout. Brain Behav Evol 54:263–275. https://doi.org/10.1159/000006627
Painter MM, Buerkley MA, Julius ML, Vajda AM, Norris DO, Barber LB, Furlong ET, Schultz MM, Schoenfuss HL (2009) Antidepressants at environmentally relevant concentrations affect predator avoidance behavior of larval fathead minnows (Pimephales promelas). Environ Toxicol Chem 28:2677–2684. https://doi.org/10.1897/08-556.1
Parrott JL, Metcalfe CD (2018) Nest-defense behaviors in fathead minnows after lifecycle exposure to the antidepressant venlafaxine. Environ Pollut. https://doi.org/10.1016/j.envpol.2017.11.049
Parrott JL, Metcalfe CD (2017) Assessing the effects of the antidepressant venlafaxine to fathead minnows exposed to environmentally relevant concentrations over a full life cycle. Environ Pollut. https://doi.org/10.1016/j.envpol.2017.06.009
Pollard D, Russel B, Carpenter K, Iwatsuki Y, Vega-Cendejas M, Jassim Kawari A, Hartmann S, Alnazry H, Abdulqader E, Alam S, Bishop J, Hassan-Al-Khalf K, Kaymaram F (2014) Diplodus sargus [WWW Document]. IUCN Red List Threat Species. https://doi.org/10.2305/IUCN.UK.20143.RLTS.T170155A42736975.en
Rodríguez-Mozaz S, Álvarez-Muñoz D, Barceló D (2017) Pharmaceuticals in the marine environment: analytical techniques and applications. In: Environmental problems in marine biology: methodological aspects and applications. Taylor & Francis Publisher, CRC Press, Abingdon, UK, pp 268–316. https://doi.org/10.1201/9781315119113
Rönnegård L, Shen X, Alam M (2010) hglm: a package for fitting hierarchical generalized linear models. R J 2:20–28
Santos LHMLM, Gros M, Rodriguez-Mozaz S, Delerue-Matos C, Pena A, Barceló D, Montenegro MCBSM (2013) Contribution of hospital effluents to the load of pharmaceuticals in urban wastewaters: Identification of ecologically relevant pharmaceuticals. Sci Total Environ 461–462:302–316. https://doi.org/10.1016/j.scitotenv.2013.04.077
Schlüsener MP, Hardenbicker P, Nilson E, Schulz M, Viergutz C, Ternes TA (2015) Occurrence of venlafaxine, other antidepressants and selected metabolites in the Rhine catchment in the face of climate change. Environ Pollut. https://doi.org/10.1016/j.envpol.2014.09.019
Schultz MM, Furlong ET (2008) Trace analysis of antidepressant pharmaceuticals and their select degradates in aquatic matrixes by LC/ESI/MS/MS. Anal Chem 80:1756–1762. https://doi.org/10.1021/ac702154e
Schultz MM, Painter MM, Bartell SE, Logue A, Furlong ET, Werner SL, Schoenfuss HL (2011) Selective uptake and biological consequences of environmentally relevant antidepressant pharmaceutical exposures on male fathead minnows. Aquat Toxicol 104:38–47. https://doi.org/10.1016/j.aquatox.2011.03.011
Sebire M, Elphinstone Davis J, Hatfield R, Winberg S, Katsiadaki I (2015) Prozac affects stickleback nest quality without altering androgen, spiggin or aggression levels during a 21-day breeding test. Aquat Toxicol. https://doi.org/10.1016/j.aquatox.2015.09.009
Sehonova P, Plhalova L, Blahova J, Doubkova V, Marsalek P, Prokes M, Tichy F, Skladana M, Fiorino E, Mikula P, Vecerek V, Faggio C, Svobodova Z (2017) Effects of selected tricyclic antidepressants on early-life stages of common carp (Cyprinus carpio). Chemosphere 185:1072–1080. https://doi.org/10.1016/j.chemosphere.2017.07.092
Sehonova P, Svobodova Z, Dolezelova P, Vosmerova P, Faggio C (2018) Effects of waterborne antidepressants on non-target animals living in the aquatic environment: a review. Sci Total Environ. https://doi.org/10.1016/j.scitotenv.2018.03.076
Stara A, Bellinvia R, Velisek J, Strouhova A, Kouba A, Faggio C (2019) Acute exposure of common yabby (Cherax destructor) to the neonicotinoid pesticide. Sci Total Environ 665:718–723. https://doi.org/10.1016/j.scitotenv.2019.02.202
Team RCD (2018) R Development Core Team. R A Lang Environ Stat Comput. https://doi.org/http://www.R-project.org
Weinberger J, Klaper R (2014) Environmental concentrations of the selective serotonin reuptake inhibitor fluoxetine impact specific behaviors involved in reproduction, feeding and predator avoidance in the fish Pimephales promelas (fathead minnow). Aquat Toxicol. https://doi.org/10.1016/j.aquatox.2013.10.012
Winberg S, Nilsson GE (1993) Roles of brain monoamine neurotransmitters in agonistic behaviour and stress reactions, with particular reference to fish. Comp Biochem Physiol Part C Pharmacol Toxicol Endoc. https://doi.org/10.1016/0742-8413(93)90216-8
Acknowledgements
The authors would like to acknowledge all colleagues who contributed to the success of the present study, namely, Catarina Santos, Cátia Figueiredo, Luana Micarelli and Miguel Baptista.
Funding
This study was funded by Portuguese national funds through FCT—Fundação para a Ciência e Tecnologia, I.P., within the strategic project UID/MAR/04292/2013 granted to MARE. FCT also supported this study through a PhD scholarship to JRP (SFRH/BD/111153/2015) and Programa Investigador FCT 2013 – Development Grant to RR.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
The authors declare that all applicable international, national and institutional guidelines for the care and use of the animals used in this study were strictly followed. This article does not contain any studies with human participants performed by any of the authors.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Rodrigues, A., Borges, F.O., Pissarra, V. et al. First indication of deleterious impacts in white-seabream larvae (Diplodus sargus) survival and behaviour following acute venlafaxine exposure. Ecotoxicology 28, 612–618 (2019). https://doi.org/10.1007/s10646-019-02057-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-019-02057-7