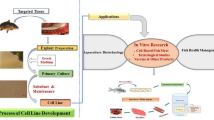
Abstract
The primary culture of fish gill cells can provide functional, cell diverse, model in vitro platforms able to tolerate an aqueous exposure analogous to in vivo tissues. The utility of such models could be extended to a variety of longer term exposure scenarios if a method could be established to extend culture viability when exposed to water for longer periods. Here we report findings of a series of experiments to establish increased longevity, as monitored by culture transepithelial electrical resistance (TEER) and concurrent histological developments. Experimental cultures improved TEER during apical freshwater exposure for a mean of twelve days, compared to previous viabilities of up to 3 days. Cultures with larger surface areas and the use of trout serum rather than foetal bovine serum (FBS) contributed to the improvement, while perfusion of the intact gill prior to cell harvest resulted in a significantly faster preparation. Detailed scanning electron microscopy analysis of cultures revealed diverse surface structures that changed with culture age. Cultures grown on membranes with an increased porosity, collagen coating or 3D structure were of no benefit compared to standard membranes. Increased culture longevity, achieved in this study and reported for the first time, is a significant breakthrough and opens up a variety of future experimentation that has previously not been possible. The extended viability facilitates exploration of in vitro chronic or pulse-exposure test paradigms, longer term physiological and environmental monitoring studies and the potential for interactive co-culture with other organoid micro-tissues.








Similar content being viewed by others
References
Alper SJ, Bronikowski AM, Harper JM (2015) Comparative cellular biogerontology: where do we stand? Exp Gerontol 71:109–11
Baron MG, Purcell WM, Jackson SK, Owen SF, Jha AN (2012) Towards a more representative in vitro method for fish ecotoxicology: morphological and biochemical characterisation of three-dimensional spheroidal hepatocytes. Ecotoxicology 21:2419–2429
Baron MG, Mintram KS, Owen SF, Hetheridge MJ, Moody AJ, Purcell WM, Jackson SK, Jha AN (2017) Pharmaceutical Metabolism in Fish: Using a 3-D Hepatic In Vitro Model to Assess Clearance. PLoS ONE 12(1):e0168837
Boyle D, Fox JE, Akerman JM, Sloman KA, Henry TB, Handy RD (2014) Minimal effects of waterborne exposure to single-walled carbon nanotubes on behaviour and physiology of juvenile rainbow trout (Oncorhynchus mykiss). Aquat Toxicol 146:154–164
Bury NR, Schnell S, Hogstrand C (2014) Gill cell culture systems as models for aquatic environmental monitoring. J Exp Biol 217:639–65
Burden N, Chapman K, Sewell F, Robinson V (2015) Pioneering Better Science through the 3Rs: an introduction to the national centre for the replacement, refinement, and reduction of animals in research (NC3Rs). J Am Assoc Lab Anim Sci 54(2):198–208
Chen S, Einspanier R, Schoen J (2015) Transepithelial electrical resistance (TEER): a functional parameter to monitor the quality of oviduct epithelial cells cultured on filter supports. Histochem Cell Biol 144(5):509–515
El-Dakhly AT, Azab AES, Alrawi QK, Lashkham NM (2015) Evaluation of substitution of fetal calf serum in VERO cell cultures by fish serum. Int J Curr Res Rev 3(2):1–8
Farkas J, Christian P, Gallego-Urrea JA, Roos N, Hassellöv M, Tollefsen KE, Thomas KV (2011) Uptake and effects of manufactured silver nanoparticles in rainbow trout (Oncorhynchus mykiss) gill cells. Aquat Toxicol 101:117–125
Fletcher M, Kelly SP, Pärt P, O’Donnell MJ, Wood CM (2000) Transport properties of cultured branchial epithelia from freshwater rainbow trout: a novel preparation with mitochondria-rich cells. J Exp Biol 203:1523–1537
Fujiwara M, Tsukada R, Shioya I, Takagi M (2009) Effects of heat treatment and concentration of fish serum on cell growth in adhesion culture of Chinese hamster ovary cells. Cytotechnology 59:135–141
Fujiwara M, Aizu Y, Shioya I, Takagi M (2010) Fetal-calf-serum-free suspension culture of Chinese hamster ovary cells employing fish serum. J Biosci Bioeng 109:307–309
Galvez F, Tsui T, Wood CM (2008) Cultured trout gill epithelia enriched in pavement cells or in mitochondria-rich cells provides insights into Na+ and Ca2+ transport. In Vitro Cell Dev Biol Animal 44:415–425
Gilmour KM, Pärt P, Prunet P, Pisam M, McDonald DG, Wood CM (1998) Permeability and morphology of a cultured branchial epithelium from the rainbow trout during prolonged apical exposure to freshwater. J Exp Zool 281:531–545
Hashimoto H, Toyohara H, Yokoyama Y, Sakaguchi M, Ozato K, Wakamatsu Y (1997) Effect of carp serum on the growth of the goldfish fin cells in early passage. J Fish Biol 50:201–207
Iftikar FI, Matey V, Wood CM (2010) The ionoregulatory responses to hypoxia in the freshwater rainbow trout oncorhynchus mykiss. Physiol Biochem Zool 83(2):343–355
Jimenez AG, Van Brocklyn J, Wortman M, Williams JB (2014) Cellular metabolic rate is influenced by life-history traits in tropical and temperate birds. PLoS ONE 9(1):e87349
Kelly SP, Fletcher M, Pärt P, Wood CM (2000) Procedures for the preparation and culture of “reconstructed” rainbow trout branchial epithelia. Methods Cell Sci 22:153–163
Kelly SP, Wood CM (2001) Effect of cortisol on the physiology of cultured pavement cell epithelia from freshwater trout gills. Am J Physiol 281:R811–R820
Kelly SP, Wood CM (2002) Cultured gill epithelia from freshwater tilapia (Oreochromis niloticus): Effect of cortisol and homologous serum supplements from stressed and unstressed fish. Membrane Biol 190:29–42
Kocal T, Quinn BA, Smith IR, Ferguson HW, Hayes MA (1988) Use of trout serum to prepare primary attached monolayer cultures of hepatocytes from rainbow trout (Salmo gairdneri). In Vitro Cell Dev Biol 24:304–308
Leguen I, Cauty C, Odjo N, Corlu A, Prunet P (2007) Trout gill cells in primary culture on solid and permeable supports. Comp Biochem Physiol A Mol Integr Physiol 148:903–912
Lillicrap A, Belanger S, Burden N, Du Pasquier D, Embry MR, Halder M, Lampi MA, Lee L, Norberg-King T, Rattner BA, Schirmer K, Thomas P (2016a) Alternative approaches to vertebrate ecotoxicity tests in the 21st century: a review of developments over the last 2 decades and current status. Environ Toxicol Chem 35(11):2637–2646
Lillicrap A, Springer T, Tyler CR (2016b) A tiered assessment strategy for more effective evaluation of bioaccumulation of chemicals in fish. Regul Toxicol Pharmacol 75:20–26
Matey V, Iftikar FI, De Boeck G, Scott GR, Sloman KA, Almeida-Val VMF, Val AL, Wood CM (2011) Gill morphology and acute hypoxia: responses of mitochondria-rich, pavement, and mucous cells in the Amazonian oscar (Astronotus ocellatus) and the rainbow trout (Oncorhynchus mykiss), two species with very different approaches to the osmo-respiratory compromise. Can J Zool 89:307–324
Minghetti M, Schnell S, Chadwick MA, Hogstrand C, Bury NR (2014) A primary Fish Gill Cell System (FIGCS) for environmental monitoring of river waters. Aquat Toxicol 154:184–192
OECD (2012) Fish Toxicity Testing Framework. Series on testing and assessment no. 171, Organisation for Economic Cooperation and Development, Paris. ENV/JM/MONO(2012)16:171
Pärt P, Norrgren L, Bergström E, Sjoberg E (1993) Primary cultures of epithelial cells from rainbow trout gills. J Exp Biol 175:219–232
Pawlowski S, Islinger M, Völkl A, Braunbeck T (2000) Temperature-dependent vitellogenin-mRNA expression in primary cultures of rainbow trout (Oncorhynchus mykiss) hepatocytes at 14 and 18 °C. Toxicol In Vitro 14(6):531–40
Perry SF, Davie PS, Daxboeck C, Ellis AG, Smith DG (1984) 10 Perfusion methods for the study of gill physiology. In: Hoar WS, Randall DJ (ed) Fish Physiology Volume X: Gills, Part B: Ion and Water Transfer. Academic Press, Orlando, p 325–388
Perry SF, Laurent P (1993) Environmental effects on fish gill structure and function. In: Rankin JC, Jensen FB (ed) Fish Ecophysiology. Chapman and Hall, London, p 231–264
Perry SF (1997) The chloride cell: structure and function in the gills of freshwater fishes. Annu Rev Physiol 59:325–347
Rathore G, Sood N, Swaminathan R (2001) Primary cell culture from fish gills and kidney using fish serum. Indian J Exp Biol 39:936–938
Rosa J, Tiago DM, Dias J, Cancela ML, Laizé V (2010) Serum-specific stimulation of proliferation and mineralization of fish bone-derived cells. J Appl Ichthyol 26(2):251–256
Schnell S, Stott LC, Hogstrand C, Wood CM, Kelly SP, Pärt P, Owen SF, Bury NR (2016) Procedures for the reconstruction, primary culture and experimental use of rainbow trout gill epithelia. Nat Protoc 11:490–498
Srinivasan B, Kolli AR, Esch MB, Abaci HE, Shuler ML, Hickman JJ (2015) TEER measurement techniques for in vitro barrier model systems. J Lab Autom. 20(2):107–126
Stott LC, Schnell S, Hogstrand C, Owen SF, Bury NR (2015) A primary fish gill cell culture model to assess pharmaceutical uptake and efflux: evidence for passive and facilitated transport. Aquat Toxicol 159:127–137
Tollefsen K-E, Eikvar S, Finne EF, Fogelberg O, Gregersen IK (2008) Estrogenicity of alkylphenols and alkylated non-phenolics in a rainbow trout (Oncorhynchus mykiss) primary hepatocyte culture. Ecotoxicol Environ Saf 71:370–383
US EPA (2002) US Environmental Protection Agency. Methods for measuring the acute toxicity of effluents and receiving waters to freshwater and marine organisms, 5th ed. https://www3.epa.gov/region6/water/npdes/wet/wet_methods_manuals/atx.pdf Accessed 31 January 2017
Walker PA, Bury NR, Hogstrand C (2007) Influence of culture conditions on metal-induced responses in a cultured rainbow trout gill epithelium. Environ Sci Technol 41:6505–6513
Walker PA, Kille P, Scott A, Bury NR, Hogstrand C (2008) An in vitro method to assess toxicity of waterborne metals to fish. Toxicol 30:67–77
Weissgerber TL, Milic NM, Winham SJ, Garovic VD (2015) Beyond bar and line graphs: time for a new data presentation paradigm. PLoS Biol 13:e1002128
West GB, Woodruff WH, Brown JH (2003) Allometric scaling of metabolic rate from molecules and mitochondria to cells and mammals. Proc Nat Acad Sci 99:2473–2478
Wilson JM, Laurent P (2002) Fish gill morphology: inside out. J Exp Zool 293:192–213
Wood CM, Pärt P (1997) Cultured branchial epithelia from freshwater fish gills. J Exp Biol 200:1047–1059
Wood CM, Gilmour KM, Pärt P (1998) Passive and active transport properties of a gill model, the cultured branchial epithelium of the freshwater rainbow trout (Oncorhynchus mykiss). Comp Biochem Physiol A 119:87–96
Wood CM, Eletti B, Pärt P (2003) New methods for the primary culture of gill epithelia from freshwater rainbow trout. Fish Physiol Biochem 26:329–344
Wood CM, Kelly SP, Zhou B, Fletcher M, O’Donnell M, Eletti B, Pärt P (2002) Cultured gill epithelia as models for the freshwater fish gill. Biochim Biophys Acta 1566:72–83
Zakaria-Runkat F, Worawattanamateekul W, Lawhavinit O (2006) Production of fish serum products as substitute for fetal bovine serum in hybridoma cell cultures from surimi industrial waste. Kasetsart J (Nat Sci) 40(Suppl):198–205
Zhou BS, Kelly SP, Ianowski JP, Wood CM (2003) Effects of cortisol and prolactin on Na+ and Cl- transport in cultured branchial epithelia from FW rainbow trout. Am J Physiol Regul Integr Comp Physiol 285(6):R1305–R1316
Acknowledgements
The authors would like to thank Ben Eynon, Glenn Harper, Lynne Cooper, and Andrew Atfield (University of Plymouth) for technical support.
Funding
This work was funded by a Biotechnology and Biological Sciences Research Council (BBSRC) Research Grant IPA (BB/L01016X/1), co-funded by the AstraZeneca Global Safety, Health and Environment research programme, to ANJ supporting RJM and MGB. AstraZeneca provided support in the form of salary for SFO and grant to ANJ, but did not have any additional role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. The SFO work represents an AstraZeneca contribution in kind to the Innovative Medicines Initiative (IMI) under grant agreement no.115735—iPiE: Intelligent led assessment of Pharmaceuticals in the Environment; resources of which are composed of financial contribution from the European Union’s Seventh Framework Programme (FP7/2015-2018) and European Federation of Pharmaceutical Industries and Associations (EFPIA) companies’ in kind contribution.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
All applicable national, and/or institutional guidelines for the care and use of animals (fish) were followed.
Rights and permissions
About this article
Cite this article
Maunder, R.J., Baron, M.G., Owen, S.F. et al. Investigations to extend viability of a rainbow trout primary gill cell culture. Ecotoxicology 26, 1314–1326 (2017). https://doi.org/10.1007/s10646-017-1856-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-017-1856-6