Abstract
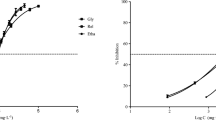
The increasing interest in the development of novel green solvents has led to the synthesis of benign alternative products with minimized environmental impacts. However, most of published studies on green solvents focus primarily on their physicochemical properties, with limited emphasis on absence of ecotoxicological assessment. In this study, we evaluated the acute ecotoxicity of four levulinates (levulinic acid, methyl levulinate, ethyl levulinate and butyl levulinate) on freshwater algae (Chlamydomonas reinhardtii), bacteria (Vibrio fischeri), daphnids (Daphnia magna) and earthworms (Eisenia foetida) using various dose–response tests. As a general trend, the toxicity of levulinate esters in aquatic exposure (assessed as the EC50) increased as a function of increasing alkyl chain length; accordingly, the most toxic compound for the aquatic organisms was butyl levulinate, followed by ethyl levulinate and methyl levulinate. The most toxic compound for E. foetida (terrestrial exposure) was methyl levulinate, followed by ethyl levulinate, butyl levulinate and levulinic acid; in this case, we observed an inverse relationship between toxicity and alkyl chain length. Based on both the lowest EC50 found in the aquatic media and the ratio between predicted environmental concentration and the predicted no-effect concentration, we have estimated the maximum allowable values in the environment for these chemicals to be 1.093 mg L−1 for levulinic acid, 2.761 mg L−1 for methyl levulinate, 0.982 mg L−1 for ethyl levulinate and 0.151 mg L−1 for butyl levulinate.






Similar content being viewed by others
References
Ahluwalia VK, Varma RS (2009) Green solvents for organic synthesis. Alpha Science International, Oxford
Almeida JRM, Modig T, Petersson A, Hahn-Hagerdal B, Liden G, Gorwa-Grauslund MF (2007) Increased tolerance and conversion of inhibitors in lignocellulosic hydrolysates by Saccharomyces cerevisiae. J Chem Technol Biotechnol 82:340–349
Anastas PT, Warner JC (1998) Green chemistry: theory and practice. Oxford University Press, Oxford and New York
Bliss CI (1934) The method of probits. Science 79:38–39
Brack W, Frank H (1998) Chlorophyll a fluorescence: a tool for the investigation of toxic effects in the photosynthetic apparatus. Ecotoxicol Environ Saf 40:34–41
Brack W, Rottler H, Frank H (1998) Volatile fractions of landfill leachates and their effect on Chlamydomonas reinhardtii: in vivo chlorophyll A fluorescence. Environ Toxicol Chem 17:1982–1991
Cho C, Jeon Y, Pham TPT, Vijayaraghavan K, Yun Y (2008) The ecotoxicity of ionic liquids and traditional organic solvents on microalga Selenastrum capricornutum. Ecotoxicol Environ Saf 71:166–171
COM (2003) 644 final de 29.10.2003. Comisión de las comunidades europeas (2003) Propuesta del Reglamento relativa al registro, la evaluación, la autorización y la restricción de las sustancias y los preparados químicos (REACH) por el que se crea la Agencia Europea de Sustancias y Preparados Químicos. CEE Bruselas
Conrad R, Buchel C, Wilhelm C, Arsalane W, Berkaloff C, Duval J (1993) Changes in yield in in-vivo fluorescence of chlorophyll a as a tool for selective herbicide monitoring. J Appl Phycol 5:505–516
Corcoll N (2011) The use of pulse amplitude modulated fluorescence techniques for metal toxicity assessment in fluvial biofilms. Dissertation. University of Girona (Spain)
Corcoll N, Bonet B, Leira M, Guasch H (2011) Chl-a fluorescence parameters as biomarkers of metal toxicity in fluvial biofilms: an experimental study. Hydrobiologia 673:119–136
Diaz-Alvarez AE, Francos J, Lastra-Barreira B, Crochet P, Cadierno V (2011) Glycerol and derived solvents: new sustainable reaction media for organic synthesis. Chem Commun 47:6208–6227
Dorigo U, Leboulanger C (2001) A pulse–amplitude modulated fluorescence-based method for assessing the effects of photosystem II herbicides on freshwater periphyton. J Appl Phycol 13:509–515
Ghose A, Crippen G (1986) Atomic physicochemical parameters for 3-dimensional structure-directed quantitative structure–activity-relationships. 1. partition-coefficients as a measure of hydrophobicity. J Comput Chem 7:565–577
Ghose A, Pritchett A, Crippen G (1988) Atomic physicochemical parameters for 3-dimensional structure directed quantitative structure-activity-relationships. 3. modeling hydrophobic interactions. J Comput Chem 9:80–90
Guasch H, Sabater S (1998) Light history influences the sensitivity to atrazine in periphytic algae. J Phycol 34:233–241
Guerrero H, Lafuente C, Royo F, Lomba L, Giner B (2011) P rho T behavior of several chemicals from biomass. Energy Fuels 25:3009–3013
Haibo X (2013) Toxicity and ecotoxicity of ionic liquids for biorefinery. In: Nicholas, Gathergood (eds) The role of green chemistry in biomass processing and conversion. Wiley, Hoboken, pp 109–115
Hutchinson T, Shillabeer N, Winter M, Pickford D (2006) Acute and chronic effects of carrier solvents in aquatic organisms: a critical review. Aquat Toxicol 76:69–92
Jennings V, Rayner-Brandes M, Bird D (2001) Assessing chemical toxicity with the bioluminescent photobacterium (Vibrio fischeri): a comparison of three commercial systems. Water Res 35:3448–3456
Johanningmeier U, Howell S (1984) Regulation of light-harvesting chlorophyll-binding protein messenger-RNA accumulation in Chlamydomonas-reinhardi: possible involvement of chlorophyll synthesis precursors. J Biol Chem 259:3541–3549
Juneau P, Dewez D, Matsui S, Kim S, Popovic R (2001) Evaluation of different algal species sensitivity to mercury and metolachlor by PAM-fluorometry. Chemosphere 45:589–598
Juneau P, El Berdey A, Popovic R (2002) PAM fluorometry in the determination of the sensitivity of Chlorella vulgaris, Selenastrum capricornutum, and Chlamydomonas reinhardtii to copper. Arch Environ Contam Toxicol 42:155–164
Keithly L, Wayne G, Cullen D, Connolly G (2005) Industry research on the use and effects of levulinic acid: a case study in cigarette additives. Nicotine Tobacco Res 7:761–771
Khusnutdinov RI, Baiguzina AR, Smirnov AA, Mukminov RR, Whemilev UM (2007) Furfuryl alcohol in synthesis of levulinic acid esters and difurylmethane with Fe and Rh complexes. Russ J Appl Chem 80:1687–1690
Lomba L, Giner B, Bandres I, Lafuente C, Pino MR (2011) Physicochemical properties of green solvents derived from biomass. Green Chem 13:2062–2070
Lomba L, Lafuente C, García-Mardones M, Gascón I, Giner B (2013) Thermophysical study of methyl levulinate. J Chem Thermodyn 65(2013):34–41
Lomize AL, Pogozheva ID, Lomize MA, Mosberg HI (2007) The role of hydrophobic interactions in positioning of peripheral proteins in membranes. BMC Struct Biol 7:44
MacPhee C, Ruelle R (1969) Lethal effects of 1888 chemicals upon four species of fish from western North America. University of Idaho (Forest, Wildlife, and Range Experiment Station), Moscow
Meylan W, Howard P (1995) Atom fragment contribution method for estimating octanol–water partition-coefficients. J Pharm Sci 84:83–92
Navarro E, Robinson CT, Behra R (2008) Increased tolerance to ultraviolet radiation (UVR) and cotolerance to cadmium in UVR-acclimatized freshwater periphyton. Limnol Oceanogr 53:1149–1158
OC SE TG 202 2004 OC SE TG 202 2004 (European C 2 method as described in the EU Regulation 440/2008)
OECD 202 (1984) Guideline for Testing of Chemicals No. 202, Daphnia sp., acute immobilisation test and reproduction test. OECD, 202, Paris, France 202
OECD 207 (1984) Guideline for Testing of Chemicals No. 207, Earthworm acute. Toxicity. OECD 207, Paris, France 207
OECD (2006) Ecological categorization results from the Canadian domestic substance list
Onorati F, Mecozzi M (2004) Effects of two diluents in the Microtox (R) toxicity bioassay with marine sediments. Chemosphere 54:679–687
Pawlisz A, Peters R (1995) Effects of sublethal exposure on lethal body burdens of narcotic organic-chemicals in daphnia-magna. Environ Sci Technol 29:613–621
QSAR Toolbox 2.3 (2009) The OECD QSAR toolbox for grouping chemicals into categories. http://www.qsartoolbox.org/download.html. Accessed 14 June 2012
Roberts B, Dorough H (1984) Relative toxicities of chemicals to the earthworm Eisenia-foetida. Environ Toxicol Chem 3:67–78
Scheringer M (2002) Persistence and spatial range of environmental chemicals: new ethical and scientific concepts for risk assessment. Wiley, Weinheim
Stoiber TL, Shafer MM, Armstrong DE (2011) Induction of reactive oxygen species in Chlamydomonas reinhardtii in response to contrasting trace metal exposures. Environ Toxicol 28(9):516–523
Szivak I, Behra R, Sigg L (2009) Metal-induced reactive oxygen species production in Chlamydomonas Reinhardtii (Chlorophyceae). J Phycol 45:427–435
Timokhin BV, Baransky VA, Eliseeva GD (1999) Levulinic acid in organic synthesis. Usp Khim 68:80–93
Tischer RG et al (1942) The non-toxicity of levulinic acid. J Am Phar Assoc 31(7):217–220
UNE-EN-ISO 11348-3 (2007) Water quality: determination of the inhibitory effect of water samples on the light emission of Vibrio fischeri (Luminescent bacteria test). UNE-EN-ISO 11348-3
Viswanadhan V, Ghose A, Revankar G, Robins R (1989) Atomic physicochemical parameters for 3 dimensional structure directed quantitative structure–activity relationships. 4. Additional parameters for hydrophobic and dispersive interactions and their application for an automated superposition of certain naturally-occurring nucleoside antibiotics. J Chem Inf Comput Sci 29:163–172
Wernet G, Conradt S, Isenring HP, Jimenez-Gonzalez C, Hungerbuehler K (2010) Life cycle assessment of fine chemical production: a case study of pharmaceutical synthesis. Int J Life Cycle Assess 15:294–303
Xiaohua Lu YH (2009) Evaluations and Toxicity of ILs. In: Mingos DMP (ed) Toxicity molecular thermodynamics of complex systems. Springer, Berlin, Heidelberg, pp 179–182
Yamada M, Hidaka T, Fukamachi H (1996) Heat tolerance in leaves of tropical fruit crops as measured by chlorophyll fluorescence. Sci Hortic 67:39–48
Acknowledgments
We would like to thank Imerys Ceramics España, S.A. for providing artificial soil for the earthworm tests. The researchers L. Lomba, B. Giner and Ma. Rosa Pino are supported by the regional Aragon Government (Consolidated Applied Research Group ref. E02) and European Social Fund “Construyendo Europa desde Aragón”. The work of S. Muñiz and E. Navarro is supported by the Spanish Ministry of Economy and Competitiveness (National Research Plan, ref. BFU2010-22053) and by the regional Aragon Government (Consolidated Applied Research Group ref. E61). Furthermore, Green Pharmacy acknowledges financial support from EEE53 SL. Business groups: Pinares de Venecia División Energética and Brial (ENATICA). Finally, we want to thank Dr. Manuel Gómez (Universidad San Jorge) both for his kind help and for having provided us with useful information.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lomba, L., Muñiz, S., Pino, M.R. et al. Ecotoxicity studies of the levulinate ester series. Ecotoxicology 23, 1484–1493 (2014). https://doi.org/10.1007/s10646-014-1290-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-014-1290-y