Abstract
In the context of warming freshwater habitats, protection of Atlantic salmon populations requires an understanding of the effects of temperature on somatic growth during the juvenile life stage. However, quantifying the effect of temperature on growth is challenging given differences among methodologies, metrics of growth, and their underlying assumptions. Using short term studies (2000–2002) in two Canadian populations of wild Atlantic salmon (Margaree and Miramichi rivers), we investigate whether different hierarchical modeling approaches influence the derivation of temperature-growth relationships, by contrasting seasonal growth trajectories (von Bertalanffy; VBGF) to size-at-age data models built with instantaneous growth rates. Size-at-age data analysed seasonally with the VBGF framework failed to detect an effect of temperature, whereas instantaneous growth rates from the same dataset were strongly related to temperature metrics. However, instantaneous growth rates cannot be used to extrapolate predictions into meaningful metrics for fisheries management (e.g., size at the end of the growing season). Nevertheless, we show that size at the end of the growing season can be predicted with VBGF models accounting for site-level variation, which in turn are related to temperature metrics, as observed for instantaneous growth rates. Taken together, these results show that combining these two approaches (size-at-age, growth rates) can circumvent their intrinsic drawbacks and reveal essential ecological patterns that may otherwise remain undetected. In cases where instantaneous growth rates are not available, relating predicted size-at-age from hierarchical VBGF to temperature provides an interesting alternative for detecting subtle environmental effects, even if the VBGF parameters or its residuals are unrelated to temperature metrics.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Atlantic salmon (Salmo salar) is important economically and culturally for Canadians and First Nations communities alike and is targeted by conservation efforts (Smialek et al. 2021; Gillis et al. 2023) due to the steady decline of populations over the past decades (ICES 2023). The juvenile life stage of salmonids is of particular concern, given the increasing frequency of thermal stress events in freshwater habitat due to climate change (Woodward et al. 2010; Breau et al. 2011; Dugdale et al. 2016; Morash et al. 2021; Smialek et al. 2021; Gallagher et al. 2022; Gillis et al. 2023). Furthermore, the juvenile life stage is critical for salmonids as they typically exhibit strong population regulation during their first summer of life (Elliott 1994; Nislow et al. 2004; Imre et al. 2005; Smialek et al. 2021; Lobon-Cervia 2022), and small reductions in body size can have long-term effects on population productivity (Koenings et al. 1993; Ulaski et al. 2022). Given the important role that somatic growth plays in salmon ecology and survival, monitoring this key parameter is essential for comprehending the potential effects and outcomes of climate change on populations.
Forecasting the effects of climate change on fitness is critical for conservation and management of Atlantic salmon (Ulaski et al. 2022; Gillis et al. 2023). A recent meta-analysis by Gallagher et al. (2022) quantified important spatial and temporal variation in salmonid productivity related to the impact of climate change on a variety of complex biological processes. However, they also underscored that the important influence of methodological and statistical techniques was challenging to ascertain in a meta-analytical framework due to substantial variation in study designs, unbalanced replication, and geographical biases. Similarly, other studies have found an important effect of study design on salmonid growth (i.e. Laplanche et al. 2019; Matte et al. 2020a), but such effects are often concomitant with potential biases induced by spatial and temporal scales (Bal et al. 2011; Gregory et al. 2017; Charron et al. 2019; Laplanche et al. 2019), and which metric/s of growth (Lugert et al. 2016) and water temperatures (Ouellet-Proulx et al. 2023) are used. Therefore, it is challenging to disentangle the potential impacts of analytical and methodological designs on reported growth-temperature relationships from other processes but warrants further investigation.
Differences in study design typically lead to different analyses due to the nature of the data collected. More specifically, investigating the effects of global climate change on salmonid growth relies on both long-term observational datasets to quantify temporal trends (Parra et al. 2009; Bal et al. 2011; Kanno et al. 2015; Laplanche et al. 2019) and short-term studies, which often include repeated measurement throughout the growth season, to investigate finer growth mechanisms and the effects of climactic extremes (e.g. Strothotte et al. 2005; Breau et al. 2011; Corey et al. 2017; Frechette et al. 2018). Both approaches provide complementary insights, but are often analyzed with different metrics of growth, and thus follow different assumptions (Lugert et al. 2016). For instance, long-term surveys without individual growth data assume that fish are sedentary, and that size-selective mortality is minimal between sampling events (Bal et al. 2011). In addition, long-term surveys conducted over larger spatial and temporal scales often rely on size-at-age data to construct seasonal growth trajectories (Parra et al. 2009; Laplanche et al. 2019; Burbank et al. 2023), usually from functions such as the von Bertalanffy Growth Function (VBGF).
Increasingly, size-at-age datasets are analysed with hierarchical modelling (e.g., when units of analysis are drawn from clusters within a population) to account for variation occurring over large spatial and temporal resolution (He and Bence 2007; Bal et al. 2011; Parent et al. 2013; Cafarelli et al. 2017; Laplanche et al. 2019). Nevertheless, an important issue is that VBGF typically models fish growth at an annual rate, even though within-season fluctuations in temperatures have a well-known influence on behavior and growth (Mallet et al. 1999; Breau et al. 2007; Dzul et al. 2017). This issue is not specific to temperature, other factors important for growth such as food availability, water discharge, or conspecific density (Heggenes 1990; Imre et al. 2005; Matte et al. 2020b) are also difficult to account for in observational designs. Consequently, the effects of temperature on growth may be more challenging to detect at a seasonal scale using VBGF models than at finer temporal resolutions.
Conversely, short-term studies on growth rates of fish that involve repeated measurement throughout the growth season are better suited to detect growth-temperature relationships (e.g. Flodmark et al. 2004; Meeuwig et al. 2004; Strothotte et al. 2005; Eldridge et al. 2015; Matte et al. 2020b). This is likely because the finer temporal resolution at which the data are collected aligns more closely with the biological processes. However, instantaneous growth rates derived from these experiments cannot be extrapolated over longer periods of time and are often calculated using the first and last measurements only, ignoring any intermediate growth measurements (Lugert et al. 2016). Furthermore, collecting growth data at time scales shorter than those required for annual size-at-age relationships is costly, labor-intensive, and often impractical (Bentley and Schindler 2013). Consequently, instantaneous growth rates are advantageous for investigating mechanisms, but pose challenges when attempting to convert them into meaningful metrics for management and conservation purposes, such as size at the end of the growing season. Despite the highlighted differences between size-at-age and instantaneous growth rates methods and their underlying assumptions, the extent to which temperature-growth relationships differ between these approaches has not received attention in the literature.
The metrics used for incorporating temperature in growth models are equally important. Historically, the effect of temperature on growth was modelled with measures of central tendency, either for air or water temperatures (Ouellet-Proulx et al. 2023). To improve these models, cumulative degree-days have also widely been used to explicitly incorporate thermal variability within a season (e.g. Strotthotte et al. 2005; Venturelli et al. 2010; Chezik et al. 2014). However, several studies failed to detect an effect of degree-days on growth (e.g. Strotthotte et al. 2005; Ouellet-Proulx et al. 2023), perhaps because the using of degree-days is equivalent to assuming a linear relationship between temperature and growth. This assumption is contradicted by experimental work on salmonids, where the relationship between growth and temperature follows a bell-shaped curve between 6 and 28 °C, with maximum growth around 16–18 °C depending on the population (Elliott et al. 1995; Mallet et al. 1999). Accordingly, Mallet et al. (1999) developed a mathematical relationship relating growth coefficients to daily temperature to estimate daily growth potential, accounting for the range of temperature at which growth occurs (hereafter referred to as growth potential in the text and subsequent analyses). The use of this growth potential metric allowed Bal et al. (2011) to detect an effect of temperature on the growth parameter K in the VBGF. To account for variation in growth season duration, Ouellet-Proulx et al. (2023) introduced the potential growth thermal index (PGTI) wherein growth potential is summed over the growth season. Studies comparing the performance of multiple temperature metrics often found that their performance is context-dependent (e.g. Charron et al. 2019; Ouellet-Proulx et al. 2023). Consequently, it is plausible that some of the idiosyncratic results reported among studies, beyond biological processes, may be related to the choice of temperature metric(s).
In the present study, we use a juvenile Atlantic salmon growth dataset collected in two eastern Canadian rivers (Margaree and Miramichi rivers) from 2000 to 2002 as a case study to investigate the potential effects of analytical and modelling decisions on the detectability of climate change effects on salmonid performance. This dataset has enough body size measurements throughout the growth season to analyze both seasonal growth trajectories and instantaneous growth rates. This approach complements recent meta-analyses (Gallagher et al. 2022) and reviews (Smialek et al. 2021; Gillis et al. 2023) as it provides an opportunity to isolate the potential effects of modelling and study designs from the important spatial and temporal biases present amongst studies, as well as providing a better understanding of the complex biological processes operating differently amongst natural systems. Using this approach, we investigate: (1) whether seasonal growth trajectories built from size-at-age data (similarly to how long-term data is analysed) differ from those derived from instantaneous growth rates while accounting for hierarchical structure at varying spatial scales (sites, populations); (2) whether these different approach influence our ability to derive temperature-growth relationships; and (3) whether combining these approaches can yield valuable insight from a management standpoint, such as predicting the size at the end of the growing season. Using our case study dataset, we test the following predictions: (1) size-at-age data will be best quantified with a VBGF using a nested spatial hierarchy (sites within populations), with a weak correlation between temperature and growth parameter K given that the former is averaged over the entire growing season; (2) temperature will be a strong driver of instantaneous growth rates, given the shorter timescale matches biological processes more closely; (3) size at the end of the growing season will be best predicted by combining insights from both size-at-age and instantaneous growth models.
Methods
Study area
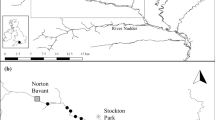
The study was conducted in two Atlantic salmon populations from eastern Canada: the Margaree River, on the western side of Cape Breton, Nova Scotia, and the Miramichi River, in New Brunswick (Fig. 1). The Margaree River drains a watershed area of 1100 km2, whereas that of the Miramichi River is approximately 12 000 km2 (Marshall 1982; Amiro 1983).
Fish Sampling methodology
Both the Margaree and Miramichi rivers were sampled using single pass backpack electrofishing following the same methodology (Strothotte et al. 2005; Dauphin et al. 2019) than the one used in the Fisheries and Oceans Canada long term monitoring program. The main discrepancy with the monitoring program being that in this study more emphasis was put in capturing a reasonable amount of fish during each sampling event to acpture the variability in size of each age category, sometimes resulting in higher effort. Sampling occurred across the stream from bank to bank in successive upstream transects. Fish were caught with hand held dip-nets and with a 1 m wide seine (1 mm mesh) downstream of each sweep (Strothotte et al. 2005). A total of 12 sites (n = 4 for Margaree River and n = 8 for Miramichi River) were repeatedly sampled (n = 5–12) throughout the growing season (Julian dates between 131 and 309, corresponding to May 10th – November 5th ) (see Table 1 for details). The Margaree River was not sampled in 2002 due to logistical constraints, but this is not expected to induce an influence on the analyses given that the year-level variation is modest (see Results). Sampling events were approximately 3 weeks apart, on average, in both populations (18.8 and 21.4 days for Margaree and Miramichi rivers, respectively). A total of 1252 and 6631 young-of-the-year (0+) salmon were caught in the Margaree and Miramichi rivers, respectively. Fork length of captured fish was measured, and age of fish was determined by visually inspecting length-frequency distributions (Sethi et al. 2017). In this analysis, only growth pattern of 0 + were investigated as they constitute the most abundant age class (42.0% of all samples) and are the only age consistently estimated across datasets (i.e., when aging juveniles based on length frequency, the overlap in size is larger between 1 + and 2 + than between 0 + and 1+). Further, the effect of environmental conditions is assumed to be more important for 0 + than older parr (Parra et al. 2012).
Temperature metrics
Water temperature was monitored using VEMCO data loggers installed at each site, which recorded data hourly (± 0.1 °C). Missing data were estimated with a Generalized Additive model relating observed water temperature averaged daily to daily air temperature data, while accounting for seasonality with Julian date using a cyclical cubic spline given the cyclical nature of seasonal data (Caissie 2006), and a site-specific random effect (RMSE: 1.07 °C and 1.47 °C for Margaree and Miramichi rivers, respectively). Daily air temperature estimates were obtained from the ERA5 reanalysis database with a 0.25 × 0.25-degree grid resolution (Hersbach et al. 2020), which were then assigned to sampling sites based on latitude and longitude.
Using predicted daily water temperatures, growth potential was calculated as in Mallet et al. (1999). Briefly, growth potential \(\mathrm{f(}{\mathrm{T}}_{\mathrm{i}}\mathrm{)}\) is a metric ranging from 0 to 1, representing no growth and maximal growth, respectively:
where t1 and t2 represent the start and end dates, respectively, and \({\phi }_{i}\left({T}_{i,d}\right)\) represents the growth potential for a given day at an observed average daily water temperature.
Growth potential, \({\phi }_{i}\left({T}_{i,d}\right),\) is defined as:
where \({T}_{i,d}\) is the observed average daily water temperature, \(\mathrm{Tmin}\), \(\mathrm{Tmax}\), and \(\mathrm{Topt}\) are the minimum, maximum, and optimal temperature for growth, respectively. Cases where \({T}_{i,d}\)is above the maximum or below the minimum for growth are assigned a growth potential of zero (\(\mathrm{f(}{\mathrm{T}}_{\mathrm{i}}\mathrm{)}\mathrm{=0}\)). \(\mathrm{Tmin}\), \(\mathrm{Tmax}\), and \(\mathrm{Topt}\) were set at 6, 27, and 18 °C, respectively, as these values match those proposed in other studies including fish from the Miramichi River population (Breau et al. 2011; Ouellet-Proulx et al. 2023).
The potential growth thermal index (PGTI) was also calculated as in Ouellet-Proulx et al. (2023) – the key difference with the growth potential from Mallet et al. (1999) being that daily growth potentials were summed over the growth season rather than averaged, to account for changes in the number of days suitable for growth:
Lastly, cumulative degree days were calculated as the cumulative sum of daily Celsius degrees above 0, as in Strottotte et al. (2005). These four temperature metrics (air temperature, growth potential, PGTI, and degree days) were chosen because they correlate well with salmonid growth (Ouellet-Proulx et al. 2023). Additionally, they can be applied at both the seasonal scale and at a finer temporal resolution for instantaneous growth rates (e.g. weeks).
Size-at-age (during the growing season)
Hypotheses were tested by implementing nonlinear hierarchical modelling under a Bayesian framework. First, hierarchical models without temperature metrics were defined; potential drivers were then added to increase complexity of models with varying levels of hierarchy. Performance of competing models was compared using the Watanabe-Akaike information criterion, which is a more generalized metric compared to the Bayesian information criterion (WAIC, Gelman et al. 2014).
2.4.1 Parametrization of hierarchical structure
The fork lengths L of fish i from site j in river l and in year m were assumed to be normally distributed as:
where \({\sigma }_{l}^{L}\) is the standard deviation associated with the VBGF and assumed to vary across rivers, and \({\mu }_{ijlm}^{L}\) is the average predicted fork length of fish i from site j in river l and year m according to the standard parametrization of the VBGF:
where L ∞ jlm represents the asymptotic fork length for a 0 + at the end of the growth season, Kjlm is the growth rate, and T0jlm is the first day of growth at site j in river l and year m.
Due to the nature of the data used in this study, a hierarchical structure was implemented for the various parameters of the VBGF reflects the belief that these processes should be similar from one site/river to another (He and Bence 2007; Bal et al. 2011; Parent et al. 2013; Cafarelli et al. 2017; Laplanche et al. 2019). Therefore, the asymptotic fork length and first day of growth parameters are drawn from Gamma distributions with river-specific mean (\({\mu }_{l}^{L\infty }\) and \({\mu }_{l}^{T0}\), respectively) and standard deviation (\({\sigma }_{l}^{L\infty }\) and \({\sigma }_{l}^{T0}\), respectively) parameters to reflect potential difference across rivers as follows:
For estimated parameter Kjlm, a beta distribution was chosen as the value of this parameter is likely bound between 0 and 1:
where \({\mu }_{l}^{K}\) and \({\sigma }_{l}^{K}\) are the mean and standard deviation of growth rate in catchment l.
2.4.2 Incorporation of temperature metrics to the VBGF
Water temperature was added to the VBGF by relating the growth rate Kjlm to temperature metrics using a linear mixed model, taking advantage of the expected relationship between growth rate Kjlm and temperature as in previous work (Taylor 1960; Fontora and Agostino 1996; Mallet et al. 1999), Eq. 8 becomes:
where al and Zl are catchment-level slope and intercept parameters, respectively. \({T}_{jlm}\) is the observed temperature metric of interest for site j in catchment l and year m (i.e., growth potential, PGTI, or degree days), and \({\mu }_{jlm}^{K}\) and \({\sigma }_{jlm}^{K}\) are the mean and associated standard deviation of growth rate. Linear mixed models were used given that the nonlinearity of the growth-temperature relationship was explicitly included in the growth potential metric (Mallet et al. 1999) and PGTI (Ouellet-Proulx et al. 2023).
Instantaneous growth rates
Using the average size of fish during each sampling event, instantaneous growth rates were calculated between consecutive sampling events within a given site separated by at least 10 days to minimize the potential effects of measurement errors, given that fish were not individually marked. Instantaneous growth rates for any given sites displayed strong non-linear patterns and were therefore analysed using Generalized additive models (GAMs). Temperature metrics for these analyses were calculated over the same time interval used to calculate instantaneous growth rates within a given site (e.g., growth rate calculated between June 1st -20th were paired with temperature metrics also calculated between June 1st -20th within that site).
Size at the end of the growing season
Sizes at the end of the growing season (October 1st ) were estimated using the best size-at-age model, given that size in October is important for later life stages (Bal et al. 2011). These extrapolations were then related to temperature metrics with GAMs using the same hierarchical structures (i.e., catchment, site, year) as those used in the instantaneous growth rates models. For these analyses, temperature metrics were calculated over the entire growing season (May-October), given that the objective was to predict the size at the end of the growing season.
Bayesian modelling
Bayesian inference for the size-at-age models was conducted with the package nimble (v. 0.13.1) in R (v. 4.2.2), given the flexibility required to build the size-at-age models with the VBGF framework as described above (i.e., these analyses could not easily be replicated with the brms package as described below). All parameters were given weakly informative priors (Table 2) except for \({\mu }_{l}^{L\infty }\) and \({\mu }_{l}^{T0}\) which were given uniform priors ranging from 40 to 120 (mm) and 75 to 165 (Julian day), respectively, to reflect our knowledge about the date of Atlantic salmon fry emergence and the maximum size 0+ can reach (Randall 1982; Swansburg et al. 2002). All parameters were estimated using 2 parallel Markov-Chain-Monte-Carlo (MCMC) chains, with 105 iterations, a burn-in of 104, and a thinning rate of 10. Convergence of nimble models was assessed with potential scale reduction factor (Brooks and Gelman 1998, Ȓ< 1.05) and confirmed visually with caterpillar plots. Model comparison was conducted using the Watanabe-Akaike information criterion (WAIC), for which lower values indicate a better fit to the data. More parsimonious models were selected when WAIC was similar (∆WAIC < 2).
Relationships between both instantaneous growth rates and size at the end of the growing season, and temperature metrics, were strongly nonlinear and, thus, were best analysed with GAM models. The brms package (a high-level interface for Stan; Carpenter et al. 2017) was used, given that the framework to build GAMs in a Bayesian framework is already implemented. All parameters of these models were estimated from a single chain with 104 iterations (including a warmup of 1000), a thinning rate of 10 resulting in 900 values retained, as is standard with brms (Bürkner 2017). Model comparison was conducted with leave-one-out cross-validation information criterion (LOOIC), and convergence of these models were also assessed with Ȓ, as provided by Stan (Vehtari et al. 2021).
The structure of all models (Table S1) and the associated diagnostic plots (Fig. S1-S12) are available in supplementary information.
Results
Overview of temperature and growth potential among rivers
Average summer water temperatures were significantly colder in the Margaree River (11.27 ± 4.98 °C) than in the Miramichi River (13.10 ± 5.93 °C, p < 0.001) from April 1st to October 1st. Similarly, air temperature was marginally colder for the Margaree River (12.43 ± 6.12 °C) than in the Miramichi River (12.79 ± 6.40 °C; p = 0.07). These differences in water temperature led to marginally smaller growth potential in the Margaree River (Growth potential = 69.15 ± 8.8%; PGTI = 105.80 ± 13.51) than the Miramichi River (Growth potential = 76.91 ± 6.01%; PGTI = 117.67 ± 9.20; p = 0.08). Daily water temperatures followed a Gaussian curve governed by three parameters: a, the maximum temperature; b, a measure of the duration of the warm period (i.e., > 0 °C), and c the Julian date at which this maximum occurs (Daigle et al. 2019). The Miramichi River exhibited a higher peak water temperature (a = 20.20 ± 1.97 °C) occurring earlier in the season (c = 206.70 ± 6.12), but a shorter growing season (b = 57.60 ± 6.15 days) than for the Margaree River (a = 16.58 ± 2.28 °C; b = 66.07 ± 1.64, c = 217.37 ± 2.18 days; all p < 0.001). No significant differences in water temperature were detected among the three years during which the studies were conducted (p = 0.21).
VBGF model
Out of the eleven models tested, the best model (based on WAIC score and parsimony of parameters) was M2 (Table 3) and did not support the hypothesis that size-at-age is best quantified with a nested spatial hierarchy and a linear relationship between temperature and parameter growth K. More specifically, model comparison from size-at-age data revealed that the most appropriate hierarchical structure included random variation among sites and years (Fig. 2; Table 3; all Ȓ < 1.001), but no systematic differences between the two rivers’ populations (Table 3; \({\mu }^{K}\) = 0.013 mm day−1, CI: 0.011–0.015; \({\mu }^{L\infty }\) = 67.2 mm, CI: 62.5–72.7; \({\mu }^{T0}\) = 119.97, CI: 111.35–128.76). VBGF models with the same hierarchical structure were also not improved by the inclusion of any temperature metrics (M4,5,6,7 ∆WAIC < 0.1; Table 3). Furthermore, the slopes of the linear regression for growth potential (M4, 0.008, CI: -0.018–0.035), degree days (M5, 2.4e-06, CI: -3.9e-06–8.5e-06), PGTI (M6, 5.7e-05, CI: -1.2e-04–2.27e-04), and air temperature (M7, 0.0011, CI: 0.0001–0.0032) were estimated at or close to 0 for all three models (Table 3), and the standard deviation in growth rate \({\sigma }_{l}^{K}\) was slightly larger with the inclusion of temperature metrics than without (\({\sigma }_{l}^{K}\)= 0.005 and 0.004, respectively). Incorporating a nested random effect of site within river (M8, M9, M10, M11) did not improve model performance (∆WAIC > 1.5, Table 3).
Mean fork length of Atlantic salmon young of the year (0+) sampled at Miramichi and Margaree rivers. Each colour indicates a specific year, the size of the dots is proportional to the number of 0 + sampled at a particular sampling event date. Lines corresponds to the average VBGF curves (most parsimonious model, M2), and error bar is the standard deviation of fork length within a sampling event
Residuals of the most parsimonious VBGF model (M2) were unrelated to any temperature metrics, quantified either cumulatively from the start of the season to the sampling date or calculated over the entire summer season (Fig. 3). Taken together, these results suggest important site-level variation in size-at-age (Table 4), but no detectable relationship between temperature metrics and the growth parameter K of the VBGF model in these populations.
Residuals of the most parsimonious size-at-age model (M2) in relation to temperature metrics (A, E: Potential Growth Thermal Index (PGTI); B, F: growth potential; C,G: Degree days); D, H: Air temperature (°C), quantified either cumulatively from the start of the season to the sampling date (A - D) or seasonally (E-H). Average residual is 0.00027
Instantaneous growth rates model
The models investigated in this study support our prediction that temperature is a strong driver of instantaneous growth rates, as the short timescale aligns more closely aligns with biological processes. The best model for growth rate (M12, ∆LOOIC < 3.3; Table 5) described a nonlinear relationship with Julian day in which the highest growth rate occurred in mid-July (Ȓ < 1.001; Fig. 4a), and a positive relationship with growth potential (Ȓ < 1.001; Fig. 4b). Accounting for potential hierarchical structure did not improve the model – no systematic differences were found among sites, populations, or sites nested within populations (M15, M16, M17, M18, ∆ LOOIC < -3.3; Table 5). The gradient of instantaneous growth rate ranged from − 0.001 (95% CI: -0.008-0.0065) to 0.011 mm.day−1 (95% CI: 0.005–0.018), from the lowest (10%) and highest (99.8%) growth potential, respectively.
Effect of (A) growth potential and (B) Julian day on instantaneous growth rates (M10 fit) of 0 + Atlantic salmon in two populations (blue: Margaree River; red: Miramichi River; dot size is proportionate to the number of days between sampling events at a given site). Note: in some case the instantaneous growth rate is negative and reflects sampling variation at a particular site this likely does not reflect a negative growth of individuals
Size at the end of the growing season
The model investigated in this study also supports our hypothesis that size at the end of the growing season will be best predicted by combining insights from both size-at-age and instantaneous growth rates models. Size at the end of the growing season as estimated by the best hierarchical VBGF model (M2) was not different among populations (Margaree River: 57.3 ± 7.5 mm; Miramichi River: 53.6 ± 5.4 mm; p = 0.30) or across years (p = 0.36). Instead, size at the end of the growing season was positively related to air temperature (M29, Table 6; Fig. 5a, LOOIC = 143.54), PGTI (M33; Table 6; Fig. 5c; ΔLOOIC < 1), and growth potential (M34; Table 6; Fig. 5b; ΔLOOIC < 1). Size at the end of the growing season increased from 47.6 mm (CI: 32.6–63.0) at 13.5 ◦C to 56.3 mm at 15.3 ◦C (M28; Fig. 5). Similarly, M33 predicted a 3.7 mm increase in size from 53.5 mm (CI: 39.7–68.0) to 57.2 mm (CI: 41.6–73.0), approximately 7.0% of fork length across the gradient of seasonal growth potential due to temperature (62–91%; Fig. 5). Models incorporating differences among rivers (M28, M30, M31, M35) performed equally well, but were less parsimonious (ΔLOOIC < -2). Conversely, models with degree days (M37; Fig. 5d) worse than the ones using other temperature metrics (ΔLOOIC > 2) and showed no clear relationship. These results suggest that the relationship between size at the end of the growing season and temperature was not different among rivers.
Discussion
Modelling mechanisms underpinning the effects of climate change on juvenile salmonid growth is challenging given the large range of spatiotemporal scales, metrics of temperature and growth, and statistical analyses investigated across studies. Here, size-at-age data analysed seasonally with the VBGF framework failed to detect an influence of temperature on growth parameters or residuals. Conversely, instantaneous growth rates between sampling dates derived from the same dataset were strongly related to growth potential, PGTI, air temperature, and even degree days to a lesser extent. However, the instantaneous growth rates models have a poor predictive capacity which limits their relevancy for population management purposes. Size at the end of the growing season was best predicted from the VBGF model accounting for random variation among sites and years and were strongly related to temperature metrics (air temperature, growth potential, PGTI) such as instantaneous growth rate models.
Here, we show that combining these two approaches (i.e., size-at-age and instantaneous growth rates modelling) can circumvent their intrinsic drawbacks and reveal essential patterns for population management that may otherwise remain undetected if only one approach was used. Further, in cases where instantaneous growth rates are not available to investigate finer patterns, relating size-at-age predicted from hierarchical VBGF can provide an interesting alternative, even if the VBGF parameters or its residuals are unrelated to temperature metrics.
Relationship between growth parameter K and temperature
The lack of relationship between the VBGF growth parameter K and temperature metrics contrasts with previous work in which such relationships are evident (Taylor 1960; Fontoura and Agostinho 1996; Mallet et al. 1999; Bal et al. 2011). An important difference is that the current study was conducted in the summer whereas Mallet et al. (1999) was conducted throughout the year, resulting in a larger gradient of temperatures and growth rates in the latter. Nevertheless, the range of growth potential among sites (62–91%) in this study was larger than in Bal et al. (2011; 72–83%), in which a small effect of temperature on growth parameter K was detected, in part due to strong density effects. Thus, it appears unlikely that our range of growth potential was too small to detect the expected relationship.
The most likely explanation is that the VBGF is not sensitive enough to detect statistically significant relationships. More specifically, VBGF parameters were usually correlated, especially growth K and asymptotic length L∞, as is common for VBGF models (Pauly 1980). Thus, small changes in growth parameter K due to temperature fluctuations are likely compensated by the other parameters (L∞, T0) during parameter estimation, exacerbating the difficulty in detecting these relationships. Taken together, results suggest that relating growth parameter K to temperature metrics may fail to detect subtle underlying growth-temperature relationship.
Relationship between instantaneous growth rates and temperature
In contrast, instantaneous growth rates calculated from the same dataset were strongly related to temperature metrics, as commonly observed in salmonids (Jensen 2003; Jonsson and Jonsson 2009). Interestingly, growth potential performed better than PGTI for instantaneous growth models. One likely explanation is that PGTI, which is summed over a given time interval, is less appropriate to quantify an average growth rate from variable time intervals compared to growth potential, which is also averaged over the time interval. The nonlinear relationship between instantaneous growth rate and Julian date peaked in early summer, which is also quite common in salmonids (e.g., Rossi et al. 2022). The two negative outliers (Fig. 4a) are likely caused by sampling variation occurring early in the season. These results differ from the VBGF model which did not detect an effect of temperature on growth rate, supporting the assertion that growth quantified at a finer temporal resolution better reflect biological processes (Mallet et al. 1999; Dzul et al. 2017).
Results also differ from a previous analysis of the same dataset for the Margaree River, which found no relationship between growth rates and temperature in 0 + juveniles (Strothotte et al. 2005). The key difference with the analysis published in Strothotte et al. (2005) is the inclusion of growth potential and PGTI, which are metrics that are more biologically relevant than degree days to quantify growth (Mallet et al. 1999).
Size at the end of the growing season
Size at the end of the growing season, as predicted by the best VBGF model (M2), was strongly related to air temperature, growth potential and PGTI, suggesting that the influence of temperature was easier to detect as a cumulative effect of daily variation over the entire growing season, after accounting for site-level variation, than through its influence on the VBGF growth parameter K. This is similar to Bal et al. (2011), which found a subtle but significant effect of temperature on the fork length reached by 0 + in October. Conversely, using degree days as a temperature metric did not find a clear relationship.
The gradient in size at the end of the growing season for 0 + in both rivers attributable to air temperature and growth potential, calculated as the maximum minus minimum size on October 1st across the range of values of the temperature metric, was 8.7 mm and 3.4 mm, respectively. These reductions are comparable to long-term studies on the impact of climate change on juvenile Atlantic salmon. Ryan et al. (2023) found a ~ 5 mm fork length reduction in 1 + parr (~ 4% of total length) in East Macchias and Sheepscot populations (air temperature: 15.7–19.7 °C), whereas Bal et al. (2011) described a slightly larger size variation of 0 + juveniles (~ 11 mm, 12% of total length) in a Normandy population (water temperature: 13.6–16.3 °C).
Hierarchical structure
When fitting fork length data to VBGF models, the most parsimonious structure only accounted for random variation among sites and years, without systematic differences among populations. This suggests important spatial heterogeneity within population, likely related to important site-level variation in terms of temperature patterns across different branches (Swansburg et al. 2002), fish density (Imre et al. 2005), and/or habitat productivity. However, no consistent differences were detected between populations, either in growth trajectories or size at the end of the growing season. One possible explanation is that the differences in thermal regime detected among rivers (e.g. peak daily water temperature averages: Margaree River, 16.58 ± 2.28 °C; Miramichi River, 20.20 ± 1.97 °C) occurred on either side of the optimal growth temperature (~ 18 °C degrees: Breau et al. 2007; Ouellet-Proulx et al. 2023). Ultimately, these differences in temperatures led to comparable growth potential among rivers (Margaree River, 69.15 ± 8.8%; Miramichi River, 76.91 ± 6.01%), possibly explaining the similar growth trajectories among populations. Similarly, no difference was detected in air temperature among populations (Margaree River, 12.43 ± 6.3◦C; Miramichi River, 12.79 ± 6.4◦C, p > 0.05).
In contrast, instantaneous growth rates were not explained by spatiotemporal hierarchy, suggesting that fish from different sites, rivers, or years, would exhibit comparable changes in growth rates given similar temperature fluctuations on a given Julian date. Taken together, these results suggests that seasonal growth trajectories are mostly linked to local site-level characteristics, but that fish growth rates respond similarly to daily temperature fluctuations across sites within these two populations and over the period considered (2000–2002).
Caveats
The models described in this study rely on several key assumptions. First, growth is estimated by leveraging multiple sampling events, without individually marking fish. This assumes that (1) fish have high site fidelity between sampling events, (2) size-selective mortality does not occur between sampling events (Bal et al. 2011) and (3) all fish have the same proability of capture througout the duration of the study. It is likely that these assumptions are met in the current study. Atlantic salmon can drift downstream over a significant distance post-emergence (Eisenhauer et al. 2021), but they are mostly territorial during the first summer of life (Steingrimsson and Grant 2008). Furthermore, evidence for size-selective mortality in juvenile Atlantic salmon suggests that it is either weak, or variable across seasons or years (Einum and Fleming 2000; Good et al. 2001; Letcher and Horton 2008). For example, size-selective mortality in the first summer of life can occur under extreme climactic events such as droughts or floods (Good et al. 2001), but to the authors knowledge no such event occurred in the current study. Additionally, it is worth noting that juvenile Atlantic salmon can disperse during high temperature events (Corey et al. 2023) but this behavior has only been observed for older juveniles (1 + and 2+) and 0 + do not seem to exhibit this behavior (Breau et al. 2007). Furthermore, there is evidence that the probability of capture may vary with the date of sampling depending on the age of the juvenile (e.g., 0 + have lower probabilities of capture earlier in the year, see Dauphin et al. 2019), this may due to differential response to the electric field and/or different habitat use: young 0 + closer to the riverbed might get stuck in the gravel when entering electronarcosis making them more difficult to net. However, this bias should be systematic across sites and affecting mainly the sampling events early in the growth season and should be compensated by the large number of sampling event occurring for any given site. Finally, factors important for growth beyond temperature, such as food availability, water discharge, or conspecific density (Heggenes 1990; Imre et al. 2005; Matte et al. 2020b) are currently modelled indirectly within year- and site-level variation. Data associated with these factors were not available for the current study and could not be explicitly accounted for. When available, the inclusion of such data could provide additional insight, and may improve our ability to detect temperature-growth relationship.
The relationships between temperature metrics and growth identified in this study are quantified from among-site variation over only three years, rather than long-term temporal trends. Nevertheless, the magnitude of temperature fluctuations among sites were comparable to those in long-term climate change studies. Rates of warming in eastern Canadian rivers is approximately 1.3 °C over the past 50 years (Swansburg et al. 2002), whereas our dataset exhibited about 4 °C difference in peak temperature across rivers, and a standard deviation of ~ 2 °C across measurements within rivers. While this work focused on a finer spatial and temporal resolution, future work should incorporate broader spatiotemporal scales, and incorporate other important drivers of growth beyond temperature (Heggenes 1990; Imre et al. 2005). Furthermore, growth measurement at an individual level obtained (e.g., through scales and/or otoliths; Wright et al. 1990) by collecting fish at the end of the summer/growing season may provide additional insight.
Management implications
The VBGF is amongst the most widely used model in stock assessments providing science advice to inform fisheries management. Despite consideration of potential hierarchical structure, the VBGF model relating growth parameter K to temperature failed to detect the expected growth-temperature relationship. The influence of temperature on growth was detected with instantaneous growth rate, but the use of instantaneous growth rates to inform fisheries management is difficult because growth rates cannot be used for predictions (Lugert et al. 2016). In the present study, we show that taking advantage of the hierarchical VBGF’s predictive power to estimate size at the end of the growing season and combining these predictions to temperature metrics can detect the expected growth-temperature relationships. This methodology is particularly useful in long-term monitoring programs with size-at-age data, and/or for monitoring data-poor fisheries.
Specifically, identifying changes in size-at-age in 0 + salmonids could be a tool to monitor demographic changes occurring at a population scale. In Atlantic salmon, freshwater age is mainly determined by the duration of the growth season and will affect the age at smoltification (Thorpe 1986; Metcalfe 1998; Jonsson and Jonsson 2007) and furthermore, maturation (developmental, physiological, morphological, and behavioral processes leading to reproductive capacity) takes place over the entire lifecycle of Atlantic salmon (Mobley et al. 2021). In some populations, there is evidence of evolutionary trade-offs for parr choosing to stay in freshwater to mature early versus migrating to sea (Buoro et al. 2012). The age at smoltification has consequences on returning adults since salmon smoltifying at an earlier age tend to have a higher reproductive fitness (Mobley et al. 2020) but are exposed to higher predation (McCormick et al. 1998).
Applying this method to the Miramichi and Margaree rivers over the 2000–2002 period showed no consistent differences in growth trajectories among populations, despite significantly different temperature regimes. Instead, we found important site-level variation in fish size linked to growth potential. These results differ from previously published analyses on the same dataset that showed no effect of degree days on growth in 0+, highlighting the importance of incorporating more biologically relevant predictors such as growth potential (Mallet et al. 1999) and PGTI (Ouellet-Proulx et al. 2023).
Data availability
Data will be made available on the repository Dryad after acceptance of the manuscript.
References
Amiro P (1983) Aerial photographic measurement of Atlantic salmon habitat of the Miramichi River, New Brunswick. DFO Canadian Science Advisory Secretariat Science Response 83(74)
Bal G, Rivot E, Prévost E, Piou C, Baglinière JL (2011) Effect of water temperature and density of juvenile salmonids on growth of young-of‐the‐year Atlantic salmon Salmo salar. J Fish Biol 78(4):1002–1022. https://doi.org/10.1111/j.1095-8649.2011.02902.x
Bentley KT, Schindler DE (2013) Body condition correlates with instantaneous growth in stream-dwelling rainbow trout and arctic grayling. Trans Am Fish Soc 142(3):747–755. https://doi.org/10.1080/00028487.2013.769899
Breau C, Weir LK, Grant JW (2007) Individual variability in activity patterns of juvenile Atlantic salmon (Salmo salar) in Catamaran Brook, New Brunswick. Can J Fish Aquat Sci 64(3):486–494. https://doi.org/10.1139/f07-026
Breau C, Cunjak RA, Peake SJ (2011) Behaviour during elevated water temperatures: can physiology explain movement of juvenile Atlantic salmon to cool water? J Anim Ecol 80(4):844–853. https://doi.org/10.1111/j.1365-2656.2011.01828.x
Brooks SP, Gelman A (1998) General methods for monitoring convergence of iterative simulations. J Comput Graphical Stat 7(4):434–455. https://doi.org/10.1080/10618600.1998.10474787
Buoro M, Gimenez O, Prévost E (2012) Assessing adaptive phenotypic plasticity by means of conditional strategies from empirical data: the latent environmental threshold model. Evolution 66(4):996–1009. https://doi.org/10.1111/j.1558-5646.2011.01484.x
Burbank J, McDermid JL, Turcotte F, Rolland N (2023) Temporal variation in Von Bertalanffy growth curves and generation time of southern gulf of st. Lawrence spring and fall spawning Atlantic herring (Clupea harengus). Fishes 8(4):205. https://doi.org/10.3390/fishes8040205
Bürkner PC (2017) Brms: an R Package for bayesian Multilevel models using Stan. J Stat Softw 80:1–28. https://doi.org/10.18637/jss.v080.i01
Cafarelli B, Calculli C, Cocchi D, Pignotti E (2017) Hierarchical non-linear mixed-effects models for estimating growth parameters of western Mediterranean solitary coral populations. Eco Model 346:1–9. https://doi.org/10.1016/j.ecolmodel.2016.12.015
Caissie D (2006) The thermal regime of rivers: a review. Freshw Bio 51(8):1389–1406. https://doi.org/10.1111/j.1365-2427.2006.01597.x
Carpenter B, Gelman A, Hoffman MD, Lee D, Goodrich B, Betancourt M, Brubaker MA, Guo J, Li P, Riddell A (2017) Stan: a probabilistic programming language. J Stat Softw 76(1):1–32. https://doi.org/10.18637/jss.v076.i01
Charron C, St-Hilaire A, Boyer C, Ouarda TBMJ, Daigle A, Bergeron NE (2019) Regional analysis and modelling of water temperature metrics for Atlantic salmon (Salmo salar) in Eastern Canada. INRS Sci Rep 1855:1–29
Chezik KA, Lester NP, Venturelli PA (2014) Fish growth and degree-days I: selecting a base temperature for a within-population study. Can J Fish Aquat Sci 71(1):47–55. https://doi.org/10.1139/cjfas-2013-0295
Corey E, Linnansaari T, Cunjak RA, Currie S (2017) Physiological effects of environmentally relevant, multi-day thermal stress on wild juvenile Atlantic salmon (Salmo salar). Conserv Physiol 5(1):1–13. https://doi.org/10.1093/conphys/cox014
Corey E, Linnansaari T, Cunjak RA (2023) High temperature events shape the broadscale distribution of juvenile Atlantic salmon (Salmo salar). Freshw Bio 68(3):534–545. https://doi.org/10.1111/fwb.14045
Daigle A, Boyer C, St-Hilaire A (2019) A standardized characterization of river thermal regimes in Québec (Canada). J Hydrol 577:1–10. https://doi.org/10.1016/j.jhydrol.2019.123963
Dauphin GJ, Chaput G, Breau C, Cunjak RA (2019) Hierarchical model detects decadal changes in calibration relationships of single-pass electrofishing indices of abundance of Atlantic salmon in two large Canadian catchments. Can J Fish Aquat Sci 76(4):523–542. https://doi.org/10.1139/cjfas-2017-0456
Dugdale SJ, Franssen J, Corey E, Bergeron NE, Lapointe M, Cunjak RA (2016) Main stem movement of Atlantic salmon parr in response to high river temperature. Ecol Freshw Fish 25(3):429–445. https://doi.org/10.1111/eff.12224
Dzul MC, Yackulic CB, Korman J, Yard MD, Muehlbauer JD (2017) Incorporating temporal heterogeneity in environmental conditions into a somatic growth model. Can J Fish Aquat Sci 74(3):316–326. https://doi.org/10.1139/cjfas-2016-0056
Einum S, Fleming IA (2000) Selection against late emergence and small offspring in Atlantic salmon (Salmo salar). Evolution 54(2):628–639. https://doi.org/10.1111/j.0014-3820.2000.tb00064.x
Eisenhauer ZJ, Christman PM, Matte JM, Ardren WR, Fraser DJ, Grant JW (2021) Revisiting the restricted movement paradigm: the dispersal of Atlantic salmon fry from artificial redds. Can J Fish Aquat Sci 78(4):493–503. https://doi.org/10.1139/cjfas-2020-016
Eldridge WH, Sweeney BW, Law JM (2015) Fish growth, physiological stress, and tissue condition in response to rate of temperature change during cool or warm diel thermal cycles. Can J Fish Aquat Sci 72(10):1527–1537. https://doi.org/10.1139/cjfas-2014-0350
Elliott JM (1994) Quantitative ecology and the brown trout. Oxford University Press, Oxford
Elliott J, Hurley M, Fryer R (1995) A new, improved growth model for brown trout, Salmo trutta. Funct Ecol 9(2):290–298. https://doi.org/10.2307/2390576
Flodmark L, Vøllestad L, Forseth T (2004) Performance of juvenile brown trout exposed to fluctuating water level and temperature. J Fish Biol 65(2):460–470. https://doi.org/10.1111/j.0022-1112.2004.00463.x
Fontoura NF, Agostinho AA (1996) Growth with seasonally varying temperatures: an expansion of the Von Bertalanffy growth model. J Fish Biol 48(4):569–584. https://doi.org/10.1111/j.1095-8649.1996.tb01453.x
Frechette DM, Dugdale SJ, Dodson JJ, Bergeron NE (2018) Understanding summertime thermal refuge use by adult Atlantic salmon using remote sensing, river temperature monitoring, and acoustic telemetry. Can J Fish Aquat Sci 75(11):1999–2010. https://doi.org/10.1139/cjfas-2017-0422
Gallagher BK, Geargeoura S, Fraser DJ (2022) Effects of climate on salmonid productivity: a global meta-analysis across freshwater ecosystems. Glob Chang Bio 28(24):7250–7269. https://doi.org/10.1111/gcb.16446
Gelman A, Hwang J, Vehtari A (2014) Understanding predictive information criteria for bayesian models. Stat Comput 24(6):997–1016. https://doi.org/10.1007/s11222-013-9416-2
Gillis CA, Ouellet V, Breau C, Frechette D, Bergeron N (2023) Assessing climate change impacts on north American freshwater habitat of wild Atlantic salmon-urgent needs for collaborative research. Can Water Resour J 48(2):222–246. https://doi.org/10.1080/07011784.2022.2163190
Good SP, Dodson JJ, Meekan MG, Ryan DA (2001) Annual variation in size-selective mortality of Atlantic salmon (Salmo salar) fry. Can J Fish Aquat Sci 58(6):1187–1195. https://doi.org/10.1139/f01-069
Gregory SD, Nevoux M, Riley WD, Beaumont WR, Jeannot N, Lauridsen RB, Lauridsen RB, Marchand F, Scott LJ, Roussel JM (2017) Patterns on a parr: drivers of long-term salmon parr length in U.K. and French rivers depend on geographical scale. Freshw Bio 62(7):1117–1129. https://doi.org/10.1111/fwb.12929
He J, Bence J (2007) Modeling annual growth variation using a hierarchical bayesian approach and the Von Bertalanffy growth function, with application to lake trout in southern Lake Huron. Trans Am Fish Soc 136(2):318–330. https://doi.org/10.1577/T06-108.1
Heggenes J (1990) Habitat utilization and preferences in juvenile Atlantic salmon (Salmo salar) in streams. Regul Rivers 5(4):341–354. https://doi.org/10.1002/rrr.3450050406
Hersbach H, Bell B, Berrisford P, Hirahara S, Horányi A, Muñoz-Sabater J et al (2020) The era5 global reanalysis. Q J R Meteorol Soc 146(730):1999–2049. https://doi.org/10.1002/qj.3803
ICES (2023) Working group on north Atlantic salmon. ICES Sci Rep 5(41):1–478
Imre I, Grant JWA, Cunjak RA (2005) Density-dependent growth of young-of-the-year Atlantic salmon Salmo salar in Catamaran Brook, New Brunswick. J Anim Ecol 74(3):508–516. https://doi.org/10.1111/j.1365-2656.2005.00949.x
Jensen AJ (2003) Atlantic salmon (Salmo salar) in the regulated river Alta: effects of altered water temperature on parr growth. River Res Appl 19(7):733–747. https://doi.org/10.1002/rra.710
Jonsson N, Jonsson B (2007) Sea growth, smolt age and age at sexual maturation in Atlantic salmon. J Fish Biol 71(1):245–252. https://doi.org/10.1111/j.1095-8649.2007.01488.x
Jonsson B, Jonsson N (2009) A review of the likely effects of climate change on anadromous Atlantic salmon Salmo salar and brown trout Salmo trutta, with particular reference to water temperature and flow. J Fish Biol 75(10):2381–2447. https://doi.org/10.1111/j.1095-8649.2009.02380.x
Kanno Y, Letcher BH, Hitt NP, Boughton DA, Wofford JE, Zipkin EF (2015) Seasonal weather patterns drive population vital rates and persistence in a stream fish. Glob Chang Bio 21(5):1856–1870. https://doi.org/10.1111/gcb.12837
Koenings JP, Geiger HJ, Hasbrouck JJ (1993) Smolt-to-aduit survival patterns of sockeye salmon (Oncorhynchus nerka): effects of smolt length and geographic latitude when entering the sea. Can J Fish Aquat Sci 50(3):600–611. https://doi.org/10.1139/f93-06
Laplanche C, Leunda PM, Boithias L, Ardaíz J, Juanes F (2019) Advantages and insights from a hierarchical bayesian growth and dynamics model based on salmonid electrofishing removal data. Ecol Model 392:8–21. https://doi.org/10.1016/j.ecolmodel.2018.10.018
Letcher BH, Horton GE (2008) Seasonal variation in size-dependent survival of juvenile Atlantic salmon (Salmo salar): performance of multistate capture–mark–recapture models. Can J Fish Aquat Sci 65(8):1649–1666. https://doi.org/10.1139/F08-083
Lobon-Cervia J (2022) Does recruitment trigger negative density-dependent feedback loops in stream-dwelling salmonids? Can J Fish Aquat Sci 79(8):1145–1153. https://doi.org/10.1139/cjfas-2021-0055
Lugert V, Thaller G, Tetens J, Schulz C, Krieter J (2016) A review on fish growth calculation: multiple functions in fish production and their specific application. Rev Aquac 8(1):30–42. https://doi.org/10.1111/raq.12071
Mallet J, Charles S, Persat H, Auger P (1999) Growth modelling in accordance with daily water temperature in European grayling (Thymallus thymallus). Can J Fish Aquat Sci 56(6):994–1000. https://doi.org/10.1139/f99-031
Marshall T (1982) Background and management alternatives for salmon of the Margaree River: a working document for the selection of stock enhancement strategies. Fisheries and Oceans, Halifax
Matte JM, Fraser DJ, Grant JWA (2020a) Density-dependent growth and survival in salmonids: quantifying biological mechanisms and methodological biases. Fish Fish (Oxf) 21(3):588–600. https://doi.org/10.1111/faf.12448
Matte JM, Fraser DJ, Grant JWA (2020b) Population variation in density-dependent growth, mortality and their trade-off in a stream fish. J Anim Ecol 89(2):541–552. https://doi.org/10.1111/1365-2656.13124
McCormick SD, Hansen LP, Quinn TP, Saunders RL (1998) Movement, migration, and smolting of Atlantic salmon (Salmo salar). Can J Fish Aquat Sci 55(S1):77–92. https://doi.org/10.1139/d98-01
Meeuwig M, Dunham J, Hayes J, Vinyard G (2004) Effects of constant and cyclical thermal regimes on growth and feeding of juvenile cutthroat trout of variable sizes. Ecol Freshw Fish 13(3):208–216. https://doi.org/10.1111/j.1600-0633.2004.00052.x
Metcalfe NB (1998) The interaction between behavior and physiology in determining life history patterns in Atlantic salmon (Salmo salar). Can J Fish Aquat Sci 55(S1):93–103. https://doi.org/10.1139/d98-005
Mobley KB, Granroth-Wilding H, Ellmén M, Orell P, Erkinaro J, Primmer CR (2020) Time spent in distinct life history stages has sex‐specific effects on reproductive fitness in wild Atlantic salmon. Mol Ecol 29(6):1173–1184. https://doi.org/10.1111/mec.15390
Mobley KB, Aykanat T, Czorlich Y, House A, Kurko J, Miettinen A et al (2021) Maturation in Atlantic salmon ( Salmo salar , salmonidae): a synthesis of ecological, genetic, and molecular processes. Rev Fish Bio Fish 31(3):523–571. https://doi.org/10.1007/s11160-021-09656-w
Morash AJ, Speers-Roesch B, Andrew S, Currie S (2021) The physiological ups and downs of thermal variability in temperate freshwater ecosystems. J Fish Biol 98(6):1524–1535. https://doi.org/10.1111/jfb.14655
Nislow K, Einum S, Folt C (2004) Testing predictions of the critical period for survival concept using experiments with stocked Atlantic salmon. J Fish Biol 65:188–200. https://doi.org/10.1111/j.0022-1112.2004.00561.x
Ouellet-Proulx S, Daigle A, St-Hilaire A, Gillis CA, Linnansaari T, Dauphin G, Bergeron NE (2023) A potential growth thermal index for estimating juvenile Atlantic salmon (Salmo salar) size-at-age across geographical scales. J Fish Biol 103(6):1488–1500. https://doi.org/10.1111/jfb.15535
Parent E, Rivot E, Rivot E (2013) Introduction to hierarchical bayesian modeling for ecological data. CRC press Boca Raton, Florida
Parra I, Almodóvar A, Nicola G, Elvira B (2009) Latitudinal and altitudinal growth patterns of brown trout Salmo trutta at different spatial scales. J Fish Biol 74(10):2355–2373. https://doi.org/10.1111/j.1095-8649.2009.02249.x
Parra I, Almodóvar A, Ayllón D, Nicola GG, Elvira B (2012) Unravelling the effects of water temperature and density dependence on the spatial variation of brown trout (Salmo trutta) body size. Can J Fish Aquat Sci 69(5):821–832. https://doi.org/10.1139/f2012-025
Pauly D (1980) On the interrelationships between natural mortality, growth parameters, and mean environmental temperature in 175 fish stocks. ICES J Mar Sci 39(2):175–192. https://doi.org/10.1093/icesjms/39.2.175
Randall RG (1982) Emergence, population densities, and growth of salmon and trout fry in two new Brunswick streams. Can J Zool 60(10):2239–2244. https://doi.org/10.1139/z82-28
Rossi GJ, Power ME, Carlson SM, Grantham TE (2022) Seasonal growth potential of Oncorhynchus mykiss in streams with contrasting prey phenology and streamflow. Ecosphere 13(9):e4211. https://doi.org/10.1002/ecs2.4211
Ryan A, Kocik JF, Atkinson EJ, Furey NB (2023) The effects of environmental and biological factors on the length of Atlantic salmon Salmo salar age 1 + parr in three maine drainages. Trans Am Fish Soc Fish Soc 152(3):327–345. https://doi.org/10.1002/tafs.10405
Sethi SA, Gerken J, Ashline J (2017) Accurate aging of juvenile salmonids using fork lengths. Fish Res 185:161–168. https://doi.org/10.1016/j.fishres.2016.09.012
Smialek N, Pander J, Geist J (2021) Environmental threats and conservation implications for Atlantic salmon and brown trout during their critical freshwater phases of spawning, egg development and juvenile emergence. Fish Manag Ecol 28(5):437–467. https://doi.org/10.1111/fme.12507
Steingrimsson SO, Grant JW (2008) Multiple central-place territories in wild young-of-the-year Atlantic salmon Salmo salar. JAnim Ecol 77(3):448–457. https://doi.org/10.1111/j.1365-2656.2008.01360.x
Strothotte E, Chaput G, Rosenthal H (2005) Seasonal growth of wild Atlantic salmon juveniles and implications on age at smoltification. J Fish Biol 67(6):1585–1602. https://doi.org/10.1111/j.1095-8649.2005.00865.x
Swansburg E, Chaput G, Moore D, Caissie D, El-Jabi N (2002) Size variability of juvenile Atlantic salmon: links to environmental conditions. J Fish Biol 61(3):661–683. https://doi.org/10.1111/j.1095-8649.2002.tb00903.x
Taylor CC (1960) Temperature, growth, and mortality–the pacific cockle. ICES J Mar Sci 26(1):117–124. https://doi.org/10.1093/icesjms/26.1.117
Thorpe J (1986) Age at first maturity in Atlantic salmon, Salmo salar: freshwater period influence and conflicts with smolting. Can Spec Pub Fish Aquat Sci 89:7–14
Ulaski ME, Finkle H, Beaudreau AH, Westley PA (2022) Climate and conspecific density inform phenotypic forecasting of juvenile Pacific salmon body size. Freshw Bio 67(2):404–415. https://doi.org/10.1111/fwb.13850
Vehtari A, Gelman A, Simpson D, Carpenter B, Bürkner PC (2021) Rank-normalization, folding, and localization: an improved R for assessing convergence of MCMC (with discussion). Bayesian Anal 16(2):667–718
Venturelli PA, Lester NP, Marshall TR, Shuter BJ (2010) Consistent patterns of maturity and density-dependent growth among populations of walleye (Sander vitreus): application of the growing degree-day metric. Can J Fish Aquat Sci 67(7):1057–1067. https://doi.org/10.1139/F10-041
Woodward G, Perkins DM, Brown LE (2010) Climate change and freshwater ecosystems: impacts across multiple levels of organization. Philos Trans R Soc B: Biol Sci 365(1549):2093–2106. https://doi.org/10.1098/rstb.2010.0055
Wright PJ, Metcalfe NB, Thorpe JE (1990) Otolith and somatic growth rates in Atlantic salmon parr, Salmo salar L: evidence against coupling. J Fish Biol 36(2):241–249. https://doi.org/10.1111/j.1095-8649.1990.tb05599.x
Acknowledgements
We thank the Department of Fisheries and Oceans Canada field crews for collecting data, and G. Chaput for some of the data custody and early discussion. The authors thank three anonymous reviewers and Karl Phillips for their review of the original manuscript which ultimately improved the final version of this paper.
Funding
Open access funding provided by Fisheries & Oceans Canada library. The project was financially supported by the Atlantic Salmon Research Joint Venture (ASRJV), and the Mitacs program (Project - IT27996) in partnership with the Gespe’gewa’gi Institute of Natural Understanding (GINU) and the Nova Scotia Salmon Association (NSSA).
Author information
Authors and Affiliations
Contributions
JMOM: Conceptualization, Data curation, Methodology, Data analysis, Data interpretation, Writing- original draft, review, and editing.
GJRD: Conceptualization, Data curation, Methodology, Data analysis, Data interpretation, Writing - review and editing.
ASH: Data analysis, Data interpretation, Writing - review and editing.
CAG: Data interpretation, Writing - review and editing.
NEB: Data interpretation, Writing - review and editing.
CB: Conceptualization, Methodology, Data interpretation, Writing - review and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethics approval
The care and use of experimental animals complied with Canadian animal welfare laws, guidelines and policies as approved by the Department of Fisheries and Oceans Canada.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Matte, JM.O., Dauphin, G.J., St-Hilaire, A. et al. Methodological influence on detecting temperature effects on growth variability in juvenile Atlantic salmon. Environ Biol Fish (2024). https://doi.org/10.1007/s10641-024-01558-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10641-024-01558-7