Abstract
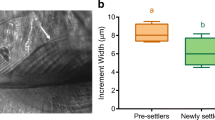
Halichoeres socialis Randall & Lobel, 2003, is the only wrasse species (Teleostei: Labridae), with a distribution confined within the Western Caribbean’s Bay of Honduras region and is most concentrated in the Meso-American Barrier Reef (MABR) lagoon of Belize. This study examined the early life history of H. socialis to gain insight into its natural history and to assess if any pattern in otolith microstructure could be linked to its biogeography. The sagittal otoliths from 67 individuals (ranging 9.8–43.4 mm SL) were analyzed to determine the daily age and increment profile. Results indicate that (1) H. socialis displays a typical “settlement mark”; (2) the pelagic larval duration (PLD) ranged from 22 to 32 (mean = 27, n = 59) days, which was similar to its congeners; and (3) the juvenile growth rate is 0.7 mm/d, which was faster than most congeners. The biogeography of H. socialis is not explained by its PLD. Alternatively, it is possible that larval behaviors (vertical migration and/or schooling) play a key factor in the distribution of H. socialis larvae.









Similar content being viewed by others
Data availability
The datasets generated from the current study are in the supplemental files. Contact the corresponding author for additional information.
References
Andradi-Brown DA, Macaya-Solis C, Exton DA, Gress E, Wright G, Rogers AD (2016) Assessing Caribbean shallow and mesophotic reef fish communities using baited-remote underwater video (BRUV) and diver-operated video (DOV) Survey Techniques. PLoS One 11:e0168235
Cowen RK, Paris CB, Srinivasan A (2006) Scaling of connectivity in marine populations. Science 311:522–527
Escobar-Sierra C, Velásquez VM, Menezes R, Rosa RS, Loaiza-Santana A (2021) An updated reef fish checklist of the southernmost Caribbean reef system, with comments on the lionfish invasion. Biota Colombiana 22:70–87
Gress E, Voss JD, Eckert RJ, Rowlands G, Andradi-Brown DA (2019) The Mesoamerican reef. In: Loya Y, Puglise KA, Bridge TCL (eds) Mesophotic coral ecosystems, Coral reefs of the world 12. Springer Cham pp 71–84
Hawes S, Miskiewicz T, Garcia V, Figueira W (2020) Size and stage-dependent vertical migration patterns in reef-associated fish larvae off the eastern coast of Australia. Deep Sea Res Part I 164:103362
Huebert KB, Cowen RK, Sponaugle S (2011) Vertical migrations of reef fish larvae in the Straits of Florida and effects on larval transport. Limnol Oceanogr 56:1653–1666
Irisson J-O, Paris CB, Guigand C, Planes S (2010) Vertical distribution and ontogenetic “migration” in coral reef fish larvae. Limnol Oceanogr 55:909–919
IUCN (2022) The IUCN red list of threatened species. Version 2022–2 https://www.iucnredlist.org (accessed 27 February 2023)
Jaxion-Harm J, Saunders J, Speight MR (2012) Distribution of fish in seagrass, mangroves and coral reefs: life-stage dependent habitat use in Honduras. Rev Biol Trop 60:683–698
Jones DL, Lara MR, Richards WJ (2005) Labridae: wrasses. In: Richards WJ (ed) Early stages of Atlantic fishes, vol II. CRC Press. Boca Raton, Fl pp, pp 1835–1872
Kramer PA, Kramer PR (2000) Ecological status of the mesoamerican barrier reef system: impacts of Hurricane Mitch and 1998 coral bleaching. Final Report to the WorId Bank #60016 (https://documents1.worldbank.org/curated/en/445151468017339734/pdf/600160ESW0Whit101public10BOX358303B.pdf)
Leis JM (1991) Vertical distribution of fish larvae in the Great Barrier Reef lagoon, Australia. Mar Biol 109:157–166
Leis JM (2006) Are larvae of demersal fishes plankton or nekton? Adv Mar Biol 51:57–141
Leis JM (2007) Behaviour as input for modelling dispersal of fish larvae: behaviour, biogeography, hydrodynamics, ontogeny, physiology and phylogeny meet hydrography. Mar Ecol Prog Ser 347:185–193
Leis JM, Hay AC, Gaither MR (2011) Swimming ability and its rapid decrease at settlement in wrasse larvae (Teleostei: Labridae). Mar Biol 158:1239–1246
Lobel PS (2011) A review of the hamlets (Serranidae, Hypoplectrus) with description of two new species. Zootaxa 3096:1–17
Lobel PS, Lobel LK (2011) Endemic marine fishes of Belize: evidence of isolation in a unique ecological region. Fish Cent Res Rep 19:48–51
Lobel PS, Rocha LA, Randall J (2009) Color phases and distribution of the western Atlantic labrid fish, Halichoeres socialis. Copeia 2009:171–174
Luiz OJ, Allen AP, Robertson DR, Floeter SR, Kulbicki M, Vigliola L, Becheler R, Madin JS (2013) Adult and larval traits as determinants of geographic range size among tropical reef fishes. Proc Natl Acad Sci 110(41):16498–16502
Mei WP (2018) basicTrendline: add trendline and confidence interval of basic regression models to plot R package version 2.0.3
Muhling BA, Smith R, Vásquez YL, Lamkin JT, Johns EM, Carrillo L, Sosa-Cordero E, Malca E (2013) Larval fish assemblages and mesoscale oceanographic structure along the Mesoamerican Barrier Reef System. Fish Oceanogr 22:409–428
Norland S, Saele Ø, Rønnestad I (2022) Developmental stages of the ballan wrasse from first feeding through metamorphosis: cranial ossification and the digestive system. J Anat 241:337–357
Panfili J, Tomás J, Morales NB (2009) Otolith microstructure in tropical fish. In: Green BS, Mapstone BD, Carlos G, Begg GA (eds) Tropical fish otoliths: information for assessment, management and ecology. Springer, Dordrecht, pp 212–248
Paris CB, Cowen RK (2004) Direct evidence of a biophysical retention mechanism for coral reef fish larvae. Limnol Oceanogr 49:1964–1979
Philibotte J (2002) Pelagic larval duration of the Caribbean wrasse, Thalassoma bifasciatum. Biol Bull 203:245–246
Potts GW, McGuigan KM (1986) Preliminary survey of the distribution of postlarval fish associated with inshore reefs and with special reference to Gobiusculus flavescens (Fabricius). Prog Underwater Science 11:15–25
Purcell JFH, Cowen RK, Hughes CR, Williams DA (2006) Weak genetic structure indicates strong dispersal limits: a tale of two coral reef fish. Proc R Soc B: Biol Sci 273:1483–1490
Purcell JFH, Cowen RK, Hughes CR, Williams DA (2009) Population structure in a common Caribbean coral-reef fish: implications for larval dispersal and early life-history traits. J Fish Biol 74:403–417
Randall JE, Lobel PS (2003) Halichoeres socialis: a new labrid fish from Belize. Copeia 2003:124–130
Raventós N, Macpherson E (2001) Planktonic larval duration and settlement marks on the otoliths of Mediterranean littoral fishes. Mar Biol 138:1115–1120
Robertson DR, Van Tassell J (2015) Shorefishes of the Greater Caribbean online information system. Version 1.0 Smithsonian Tropical Research Institute, Balboa, Panama. (accessed 16 March 2023). https://biogeodb.stri.si.edu/caribbean/en/pages/random/13298
Rocha LA, Rocha CR, Baldwin CC, Weigt LA, McField M (2015) Invasive lionfish preying on critically endangered reef fish. Coral Reefs 34:803–806
Smith CL, Tyler JC, Davis WP, Jones RS, Smith DG, Baldwin CC (2003) Fishes of the Pelican Cays, Belize. Atoll Res Bull 497:1–80
Sponaugle S, Cowen RK (1997) Early life history traits and recruitment patterns of Caribbean wrasses (Labridae). Ecol Monogr 67:177–202
Sponaugle S, Cowen RK, Shanks A, Morgan SG, Leis JM, Pineda J, Boehlert GW, Kingsford MJ, Lindeman KC, Grimes C, Munro JL (2002) Predicting self-recruitment in marine populations: biophysical correlates and mechanisms. Bull Mar Sci 70:341–375
Sweetman BM, Foley JR, Steinberg MK (2019) A baseline analysis of coastal water quality of the port Honduras marine reserve, Belize: a critical habitat for sport fisheries. Environ Biol Fish 102:429–442. https://doi.org/10.1007/s10641-018-0811-6
Vásquez-Yeomans L, Vega-Cendejas ME, Montero JL, Sosa-Cordero E (2011) High species richness of early stages of fish in a locality of the Mesoamerican barrier reef system: a small-scale survey using different sampling gears. Biodivers Conserv 2021:2379–2392
Victor BC (1982) Daily otolith increments and recruitment in two coral-reef wrasses, Thalassoma bifasciatum and Halichoeres bivittatus. Mar Biol 71:203–208
Victor BC (1986) Duration of the planktonic larval stage of one hundred species of Pacific and Atlantic wrasses (family Labridae). Mar Biol 90:317–326
Victor BC (1983) Settlement and larval metamorphosis produce distinct marks on the otoliths of the Slippery Dick, Halichoeres bivittatus. In: Reaka ML (ed) The ecology of deep and shallow coral reefs. NOAA Symposium series in undersea research, Rockville Maryland, USA, pp 47–51
Victor BC (1987) Growth, dispersal, and identification of planktonic labrid and pomacentrid reef-fish larvae in the eastern Pacific Ocean Mari Biol 95:145–152
Victor BC (2006) A photographic guide to the larvae of coral reef fishes: family Labridae. http://www.coralreeffish.com/labridae.html (accessed 19 January 2023)
Wainwright PC, Santini F, Bellwood DR, Robertson DR, Rocha LA, Alfaro ME (2018) Phylogenetics and geography of speciation in New World Halichoeres wrasses. Mol Phylogenet Evol 121:35–45
Weaver DC, Rocha LA (2007) A new species of Halichoeres (Teleostei: Labridae) from the western Gulf of Mexico. Copeia 2007:798–807
Wilson DT, McCormick MI (1999) Microstructure of settlement-marks in the otoliths of tropical reef fishes. Mar Biol 134:29–41
Acknowledgements
We thank D. Ross Robertson and an anonymous reviewer for unpublished information on biogeography. Field collection of specimens was conducted under a research permit issued by The Belize Fisheries dept. and the auspices of the Wee Caye Marine Laboratory, Belize.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
This research followed the ASIH “Guidelines for Live Amphibians and Reptiles in Field and Laboratory Research” and was conducted under a Boston University IACUC protocol.
Competing interests
Dr. Phillip Lobel is on the Editorial Board of this journal, but he was not involved in the peer review of this article and had no access to information regarding its peer review.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The field research was conducted as part of the Boston University Marine Program’s field course on coral reef fish ecology in 2001.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sun, ZX., Lobel, P.S. Pelagic larval duration of the Meso-American reef fish, Halichoeres socialis (Labridae). Environ Biol Fish 106, 1971–1982 (2023). https://doi.org/10.1007/s10641-023-01477-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10641-023-01477-z