Abstract
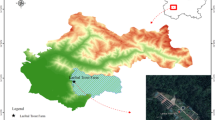
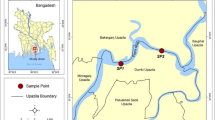
The reproductive traits of two loricariid catfishes, Ancistrus multispinis and Pareiorhaphis hypselurus, from tributaries of the Mampituba river basin, southern Brazil, were analyzed and tested for relationships with biotic and abiotic parameters. Samples were taken monthly from July 2008 to December 2009 and data were grouped by season. Traits include population structure, reproductive period, size at first maturation, fecundity, spawning type, and possible influences of biotic and abiotic factors on reproduction. Results found the sex ratio to be 1:1 for both species. Males of A. multispinis and both sexes of P. hypselurus presented isometric growth, while females of A. multispinis had positive allometric growth. The reproductive activity of both species started during winter and reached its highest intensity in spring and summer. Both species had low fecundity with large oocytes and multiple spawning. Size at first maturation for A. multispinis was 58.3 mm for males and 49.2 mm for females, while for P. hypselurus, it was 40.5 for males and 34.1 for females. Day length seems to be the most influential abiotic factor for increasing the gonadosomatic index (GSI) for both species. Other abiotic factors, such as water depth and velocity, rainfall, and temperature, may also play a role in the reproductive cycle. The hepatosomatic index (HSI) also influenced reproduction, especially for A. multispinis. The reproductive traits presented by both species, such as an extended reproductive period, large oocytes, low fecundity, multiple spawning, and the occurrence of parental care, place these species within the equilibrium reproductive strategy.






Similar content being viewed by others
Data availability
Not applicable.
Code availability
Not applicable.
Change history
19 July 2022
A Correction to this paper has been published: https://doi.org/10.1007/s10641-022-01305-w
References
Abell R, Thieme ML, Revenga C, Bryer M, Kottelat M, Bogutskaya N, Coad B, Mandrak N, Balderas SC, Bussing W, Stiassny MLJ, Skelton P, Allen GR, Unmack P, Naseka A, Ng R, Sindorf N, Robertson J, Armijo E, Higgins JV, Heibel TJ, Wikramanayake E, Olson D, López HL, Reis RE, Lundberg JG, Sabaj-Pérez MH, Petry P (2008) Freshwater ecoregions of the world: a new map of biogeographic units for freshwater biodiversity conservation. Bioscience 58(5):403–414. https://doi.org/10.1641/B580507
Adebisi AA (1987) The relationships between fecundities, gonadosomatic indices and egg sizes of some fishes of Ogun River, Nigeria. Arch Hydrobiol 111:151–156
Agostinho AA, Narahara MY, Godinho H (1982) Morfologia dos ovários de Plecostomus commersonii (Valenciennes, 1840) Osteichthyes-Loricariidae: Desenvolvimento dos ovócitos e escala de maturidade. Rev Bras Biol 42:71–77
Agostinho AA, Barbieri G, Verani JR, Hahn NS (1990) Variação do fator de condição e do índice hepatossomático e suas relações com o ciclo reprodutivo em Rhinelepis aspera (Agassis, 1829) (Osteichthyes, Loricariidae) no rio Paranapanema, Porecatu, PR. Cien Cult 42:711–714
Agostinho AA, Hahn NS, Agostinho CS (1991) Ciclo reprodutivo e primeira maturação de fêmeas de Hypostomus commersonii (Valenciennes, 1840) (Siluriformes, Loricariidae) no reservatório Capivari - Cachoeira, PR. Rev Bras Biol 51:31–37
Agostinho AA, Matsuura Y, Okada EK, Nakatani K (1995) The catfish, Rhinelepis aspera (Teleostei; Loricariidae), in the Guaíra region of the Paraná River: an example of population estimation from catch-effort and tagging data when emigration and immigration are high. Fish Res 23:333–344. https://doi.org/10.1016/0165-7836(94)00347-Y
Agostinho AA, Gomes LC, Veríssimo S, Okada EK (2004) Flood regime, dam regulation and fish in the Upper Paraná River: effects on assemblage attributes, reproduction and recruitment. Rev Fish Biol Fish 14:11–19. https://doi.org/10.1007/s11160-004-3551-y
Allan JD (1995) Stream Ecology: structure and function of running waters. Chapman & Hall, London. https://doi.org/10.1007/978-1-4020-5583-6
Andrade BN, Langeani F (2014) A new species of Neoplecostomus Eigenmann & Eigenmann 1888 (Siluriformes, Loricariidae, Neoplecostominae) from the upper Rio Paraná basin. Neotrop Ichthyol 12:675–681. https://doi.org/10.1590/1982-0224-20130195
Andrade AB, Machado LF, Hostim-Silva M, Barreiros JP (2003) Reproductive biology of the Dusky Grouper Epinephelus marginatus (Lowe, 1834). Braz Arch Biol Technol 46:373–381. https://doi.org/10.1590/S1516-89132003000300009
Antoniutti DM, Ranzani-Paiva MJT, Godinho HM (1985) Morfologia das gônadas, escala de maturidade e fator de condição de Plecostomus albopunctatus Regan, 1908 (Osteichthyes, Loricariidae) do rio Jaguari, São Paulo, Brasil. Bol Inst Pesca São Paulo 12:87–103
Armbruster JW (2003) Phylogenetic relationships of the suckermouth armoured catfishes (Loricariidae) with emphasis on the Hypostominae and the Ancistrinae. Zool J Linn Soc 141:1–80. https://doi.org/10.1111/j.1096-3642.2004.00109.x
Armbruster JW, Page LM (1996) Convergence of a cryptic saddle pattern in benthic freshwater fishes. Environ Biol Fishes 45:249–257. https://doi.org/10.1007/Bf00003092
Armbruster JW, Page LM (2006) Redescription of Pterygoplichthys punctatus and description of a new species of Pterygoplichthys (Siluriformes: Loricariidae). Neotrop Ichthyol 4(4):401–410. https://doi.org/10.1590/S1679-62252006000400003
Azevedo MA (2010) Reproductive characteristics of characid fish species (Teleostei, Characiformes) and their relationship with body size and phylogeny. Iheringia Ser Zool, Porto Alegre 100(4):469–482. https://doi.org/10.1590/S0073-47212010000400020
Azevedo MA, Malabarba LR, Fialho CB (2000) Reproductive biology of the inseminating glandulocaudine Diapoma speculiferum Cope (Teleostei: Characidae). Copeia 2000:983–989. https://doi.org/10.1643/0045-8511(2000)000[0983:RBOTIG]2.0.CO;2
Bagenal TB (1967) A short review of fish fecundity. In: Gergink D (ed) Shelby. The biological basis of freshwater fish production. Blackwell, Oxford, pp 89–111
Bailly D, Agostinho AA, Suzuki HI (2008) Influence of the flood regime on the reproduction of fish species with different reproductive strategies in the Cuiabá River, upper Pantanal, Brazil. River Res Appl 24:1218–1229. https://doi.org/10.1002/rra.1147
Bailly D, Batista-Silva VF, Abelha MCF, Kashiwaqui EAL, Fernandes CA, Carvalho ED (2011) Relative abundance and reproductive tactics of a Loricariidae species at Saraiva Lagoon, Ilha Grande National Park, MS-PR, Brazil. Biota Neotropica 11(3) https://doi.org/10.1590/S1676-06032011000300014
Bain MB, Stevenson NJ (1999) Aquatic habitat assessment: common methods. Bethesda, Maryland, Trans Am Fish Soc 224p https://doi.org/10.1046/j.1365-2400.2001.00220.x
Balon EK (1975) Reproductive guilds of fishes: a proposal and definition. J Fish Res Board Can 32:821–864. https://doi.org/10.1139/f75-110
Barbieri G (1994) Dinâmica da reprodução do cascudo Rineloricaria latirostris Boulenger (Siluriformes, Loricariidae) do Rio Passa Cinco, Ipeúna, São Paulo. Rev Bras Zool, São Paulo 11(4):600–615. https://doi.org/10.1590/S0101-81751994000400003
Barbieri G, Hartz SM, Verani JR (1996) O fator de condição e índice hepatossomático como indicadores do período de desova de Astyanax fasciatus da represa do Lobo, São Paulo (Osteichthyes, Characidae). Iheringia Ser Zool 81:97–100
Barton K (2014) MuMIn: multi-model inference. R package version 1.9.0. http://CRAN.R-project.org/package=MuMIn. Accessed 08 Apr 2020
Becker FG, Carvalho S, Hartz SM (2008) Life-history of the South American darter, Characidium pterostictum (Crenuchidae): evidence for small scale spatial variation in a piedmont stream. Neotrop Ichthyol 6(4):591–598. https://doi.org/10.1590/S1679-62252008000400007
Begg GA, Marteinsdottir G (2000) Spawning origins of pelagic juvenile cod Gadus morhua inferred from spatially explicit age distributions: potential influences on year-class strength and recruitment. Mar Ecol 202:193–217. https://doi.org/10.3354/meps202193
Benedito-Cecílio E, Agostinho AA (1997) Estrutura das populações de peixes do reservatório de segredo. In: Agostinho AA, Gomes LC (eds) Reservatório de Segredo: bases ecológicas para o manejo. Eduem, Maringá, 113–139
Blanck A, Lamouroux N (2007) Large-scale intraspecific variation in life-history traits of European freshwater fish. J Biogeogr 34:862–875. https://doi.org/10.1111/j.1365-2699.2006.01654.x
Braga FMS, Gomiero LM, Souza UP (2008) Aspectos da reprodução e alimentação de Neoplecostomus microps (Loricariidae, Neoplecostominae) na microbacia do Ribeirão Grande, serra da Mantiqueira oriental, estado de São Paulo. Acta Sci Biol Sci, Maringá 30(4):455–463. https://doi.org/10.4025/actascibiolsci.v30i4.301
Braga FMS, Gomiero LM, Souza UP (2009) Biologia populacional de Pareiorhina rudolphi (Loricariidae, Hypostominae) na microbacia do Ribeirão Grande, Serra da Mantiqueira Oriental, Estado de São Paulo. Acta Sci Biol Sci, Maringá 31(1):79–88. https://doi.org/10.4025/actascibiolsci.v31i1.459
Brito MFG, Lazzarotto H, Caramaschi EP (2016) Life-history features of a rapids-dwelling loricariid catfish from Atlantic Forest streams. Brazil Biota Neotropica 16(2):e20150068. https://doi.org/10.1590/1676-0611-BN-2015-0068
Buck S, Sazima I (1995) An assemblage of mailed catfishes (Loricariidae) in southeastern Brazil: distribution, activity, and feeding. Ichthyol Explor Freshw 6:325–332
Burgess WE (1989) An atlas of freshwater and marine catfishes. TFH Publications, Neptune City, New Jersey, A preliminary survey of the Siluriformes, p 784
Calegari BB, Lehmann PA, Reis RE (2011) A new species of Otothyropsis (Siluriformes: Loricariidae) from the rio Paraguay basin. Paraguay Neotrop Ichthyol 9(2):253–260. https://doi.org/10.1590/S1679-62252011000200002
Cantanhêde G, Castro ACL, Gubiani EA (2007) Biologia reprodutiva de Hexanematichthys proops (Siluriformes, Ariidae) no litoral ocidental maranhense. Iheringia Ser Zool 97:498–504. https://doi.org/10.1590/S0073-47212007000400021
Costa APR, Andrade DR, Vidal Junior MV, Souza G (2005) Indicadores quantitativos da biologia reprodutiva de fêmeas de piau-vermelho no Rio Paraíba do Sul. Pesqui Agropecu Bras 40:789–795. https://doi.org/10.1590/S0100-204X2005000800009
Dala-Corte RB, Azevedo MA (2010) Biologia reprodutiva de Astyanax henseli (Teleostei, Characidae) do curso superior do rio dos Sinos, RS, Brasil. Iheringia Ser Zool 100:259–266. https://doi.org/10.1590/S0073-47212010000300012
Dala-Corte RB, Fialho CB (2013) Reproductive tactics and development of sexually dimorphic structures in a stream-dwelling characid fish (Deuterodon stigmaturus) from Atlantic Forest. Environ Biol Fish 97:1119–1127. https://doi.org/10.1007/s10641-013-0202-y
De Carvalho PA, Paschoalini AL, Santos GB, Rizzo E, Bazzoli N (2009) Reproductive biology of Astyanax fasciatus (Pisces: Characiformes) in a reservoir in southeastern Brazil. J Appl Ichthyol 25(3):306–313. https://doi.org/10.1111/j.1439-0426.2009.01238.x
de Vazzoler AEAM, Menezes NA (1992) Síntese dos conhecimentos sobre o comportamento reprodutivo dos Characiformes da América do Sul (Teleostei, Ostariophysi). Rev Bras Biol 52:627–640
Dei Tós C, Agostinho AA, Suzuki HI (1997) Population structure and reproductive biology of Loricariichthys platymetopon (Siluriformes, Pisces) in the upper river Paraná. Braz Arch Biol Technol 40:793–807
Duarte S, Araújo FG, Sales A, Bazzoli N (2007) Morphology of gonads, maturity and spawning season of Loricariichthys spixii (Siluriformes, Loricariidae) in a subtropical reservoir. Braz Arch Biol Technol 50(6):1019–1032. https://doi.org/10.1590/S1516-89132007000700013
Duarte S, Araújo FG, Bazzoli N (2011) Reproductive plasticity of Hypostomus affinis (Siluriformes: Loricariidae) as a mechanism to adapt to a reservoir with poor habitat complexity. Zoologia (curitiba) 28(5):577–586. https://doi.org/10.1590/S1984-46702011000500005
Figueiredo RS, Viana LF, Moraes DA, Suarez YR (2019) Life-history traits of Farlowella hahni (Siluriformes, Loricariidae) in streams of the Ivinhema River Basin. Upper Paraná Basin Braz J Biol 79(2):286–293. https://doi.org/10.1590/1519-6984.181073
Freitas TMS, Almeida VHC, Montag LFA, Rocha RM, Fontoura NF (2011) Seasonal changes in the gonadossomatic index, allometric condition factor and sex ratio of an auchenipterid catfish from eastern Amazonia. Neotrop Ichthyol 9:839–847. https://doi.org/10.1590/S1679-62252011005000044
Fricke R, Eschmeyer WN, Van der Laan R (eds) (2022) Eschmeyer's catalog of fishes: genera, species, references. http://researcharchive.calacademy.org/research/ichthyology/catalog/fishcatmain.asp. Electronic version Accessed 03 Mar 2022
Froese R (2006) Cube law, condition factor and weight- length relationships: History, meta-analysis and recommendations. J Appl Ichthyol 22:241–253. https://doi.org/10.1111/j.1439-0426.2006.00805.x
Garutti V (1988) Distribuição longitudinal da ictiofauna em um córrego da região noroeste do Estado de São Paulo, bacia do rio Paraná. Braz J Biol 48(4):747–759
Gelain D, Fialho CB, Malabarba LR (1999) Biologia reprodutiva de Serrapinnus calliurus (Boulenger, 1900) (Characidae, Cheirodontinae) do arroio do Ribeiro, Barra do Ribeiro, Rio Grande do Sul, Brasil. Comun Mus Ciênc Tecnol PUCRS, Ser Zool 12:71–82
Godinho AL, Lamas IR, Godinho HP (2010) Reproductive ecology of Brazilian freshwater fishes. Environ Biol Fish 87:143–162. https://doi.org/10.1007/s10641-009-9574-4
Gomes ID, Araújo FG, Uehara W, Sales A (2011) Reproductive biology of the armoured catfish Loricariichthys castaneus (Castelnau, 1855) in Lajes reservoir, southeastern Brazil. J Appl Ichthyol 27:1322–1331. https://doi.org/10.1111/j.1439-0426.2011.01874.x
Gomes ID, Araújo FG, Nascimento AA, Sales A (2015) Equilibrium reproductive strategy of the armored catfish Hypostomus auroguttatus (Siluriformes, Loricariidae) in a tropical river in Southeastern Brazil. Environ Biol Fish https://doi.org/10.1007/s10641-014-0256-5
Gonçalves TK, Azevedo MA, Malabarba LR, Fialho CB (2005) Reproductive biology and development of sexually dimorphic structures in Aphyocharax anisitsi (Ostariophysi: Characidae). Neotrop Ichthyol 3(3):433–438. https://doi.org/10.1590/S1679-62252005000300012
Goulart E, Verani JR (1992) Proporção sexual, relação peso/comprimento e fator de condição de Hypostomus commersonii Valenciennes, 1840 (Osteichthyes –Loricariidae) no reservatório de Capivari-Cachoeira, Paraná, Brasil. Revista Unimar (suppl) 14:19–33
Gross MR, Sargent RC (1985) The evolution of male and female parental care in fishes. Am Zool 25:807–822
Hasenack H, Ferraro LW (1989) Considerações sobre o clima da região de Tramandaí – RS. Pesquisas (porto Alegre) 22:53–70
Hirschmann A, Fialho CB, Grillo HCZ (2011) Reprodução de Hemiancistrus punctulatus Cardoso & Malabarba, 1999 (Siluriformes: Loricariidae) no sistema da Laguna dos Patos: uma espécie de ambiente lótico frente às alterações provocadas por represamentos. Neotropical Biol Conserv 6(3):250–257. https://doi.org/10.4013/nbc.2011.63.10
Hora SL (1930) Ecology, bionomics and evolution of the torrential fauna, with special reference to the organs of attachment. Philos T R Soc Lon B Biol Sci 218:171–282. https://doi.org/10.1098/rstb.1930.0005
Isbrücker IJH (1980) Classification and catalogue of the mailed Loricariidae (Pisces, Siluriformes). Verslagen En Technische Gegevens, Universiteit Van Amsterdam 22:181
Isbrücker IJH, Nijssen H (1992) Sexualdimorphismus bei Harnischwelsen (Loricariidae). Harnischwelse. DATZ Sonderheft 19–33.
Lampert VR, Azevedo MA, Fialho CB (2004) Reproductive biology of Bryconamericus iheringii (Ostariophysi: Characidae) from rio Vacacaí, RS, Brazil. Neotrop Ichthyol 2:209–215. https://doi.org/10.1590/S1679-62252004000400003
Lampert VR, Azevedo MA, Fialho CB (2007) Reproductive biology of Bryconamericus stramineus Eigenmann, 1908 (Ostariophysi: Characidae) from the Rio Ibicuí, RS, Brazil. Braz Arch Biol Technol 50:995–1004. https://doi.org/10.1590/S1516-89132007000700011
Lizama MAP, Vazzoler AEAM (1993) Crescimento em peixes do Brasil: uma síntese comentada. Revista Unimar 15:141–173
Marcucci KMI, Orsi ML, Shibata OA (2005) Abundância e aspectos reprodutivos de Loricariichthys platymetopon em quatro trechos da represa Capivara, médio rio Paranapanema. Iheringia Ser Zool 95(2):197–203. https://doi.org/10.1590/S0073-47212005000200010
Martin-Smith KM, Armstrong JD (2002) Growth rates of wild stream-dwelling Atlantic salmon correlate with activity and sex but not Dominance. J Anim Ecol 71:413–423. https://doi.org/10.1046/j.1365-2656.2002.00609.x
Matthews WJ (1998) Patterns in freshwater fish ecology. Chapman & Hall, Massachusetts. https://doi.org/10.1007/978-1-4615-4066-3
Mazzoni R, Caramaschi EP (1995) Size, structure, sex ratio and onset of sexual maturity of two species of Hypostomus. J Fish Biol 47:841–849. https://doi.org/10.1111/j.1095-8649.1995.tb06006.x
Mazzoni R, Caramaschi EP (1997) Observations on the reproductive biology of female Hypostomus luetkeni Lacèpède 1803. Ecol Freshw Fish 6:53–56. https://doi.org/10.1111/j.1600-0633.1997.tb00143.x
Mazzoni R, Caramaschi EP, Fenerich-Verani N (2002) Reproductive biology of a characidiinae (Osteichthyes, Characidae) from the Ubatiba river, Maricá. RJ Rev Bras Biol 62(3):487–494. https://doi.org/10.1590/S1519-69842002000300013
Menezes MS, Caramaschi EP (1994) Características reprodutivas de Hypostomus grupo H. punctatus no rio Ubatiba, Maricá, RJ (Osteichthyes, Siluriformes). Rev Bras Biol 54:503–513
Mommsen TP, Korsgaard B (2008) Vitellogenesis. In: Rocha MJ, Arukwe A, Kapoor BG (eds) Fish Reproduction. New Hampshire, Science Publishers, pp 113–170 https://doi.org/10.1201/b10747
Moodie GEE, Power M (1982) The reproductive biology of an armoured catfish, Loricaria uracantha, from Central America. Environ Biol Fishes 7(2):143–148. https://doi.org/10.1007/BF00001784
Moreira AGL, Carreiro CRP, Moreira RL (2010) Eficácia do eugenol extraído da planta Eugenia aromatica como anestésico para realização de biometrias em adultos de tilápia do Nilo (Oreochromis niloticus). Acta Sci Anim Sci 32(4):419–423. https://doi.org/10.4025/actascianimsci.v32i4.9973
Myers N, Mittermeier RA, Mittermeier CG, Fonseca GAB, Kent J (2000) Biodiversity hotspots for conservation priorities. Nature 403:853–858. https://doi.org/10.1038/35002501
Nikolsky GV (1963) The ecology of fishes. Academic Press, London, 352 pp
Nomura H, Mueller IMM (1980) Biologia do cascudo Plecostomus hermanni Ihering, 1905 do Rio Mogi Guaçu, São Paulo (Osterichthyes, Loricariidae). Rev Bras Biol 40(2):267–275
Oliveira CLC, Fialho CB, Malabarba LR (2002) Período reprodutivo, desova e fecundidade de Cheirodon ibicuhiensis Eigenmann, 1915 (Ostariophysi: Characidae) do arroio Ribeiro, Rio Grande do Sul, Brasil. Comun Mus Ciênc Tecnol PUCRS, Ser Zool 15(1):3–14
Pereira EHL, Vieira F, Reis RE (2007) A new species of sexually dimorphic Pareiorhaphis Miranda Ribeiro, 1918 (Siluriformes: Loricariidae) from the rio Doce basin, Brazil. Neotrop Ichthyol 5:443–448. https://doi.org/10.1590/S1679-62252007000400003
Pereira EHL, Vieira F, Reis RE (2010) Pareiorhaphis scutula, a new species of neoplecostomine catfish (Siluriformes: Loricariidae) from the upper rio Doce basin, Southeastern Brazil. Neotrop Ichthyol 8:33–38. https://doi.org/10.1590/S1679-62252010000100005
Power ME (1984) The importance of sediment in the grazing ecology and size class interactions of an armored catfish, Ancistrus spinosus. Environ Biol Fishes 10:173–181. https://doi.org/10.1007/BF00001124
Power ME (2003) Life cycles, limiting factors and behavioral ecology of four loricariid catfishes in a Panamanian stream. pp. 581–600. In: Arratia G, Kapoor BG, Chardon M, Diogo R (eds) Catfishes, vol 2. New Hampshire, Science Publishers, Enfield, p 812
Py-Daniel LHR, Cox-Fernandes C (2005) Dimorfismo sexual em Siluriformes e Gymnotiformes (Ostariophysi) da Amazônia. Acta Amaz 35(1):97–110. https://doi.org/10.1590/S0044-59672005000100015
Querol MVM, Querol E, Gomes NNA (2002) Fator de condição gonadal, índice hepatossomático e recrutamento como indicadores do período de reprodução de Loricariichthys platymetopon (Osteichthyes, Loricariidae), bacia do rio Uruguai médio, sul do Brasil. Iheringia Ser Zool 92:79–84. https://doi.org/10.1590/S0073-47212002000300008
R Core Team (2021) R: a language and environment for statistical computing [online]. R Foundation for Statistical Computing, Vienna, Austria. Available from http://www.r-project.org/. Accessed 26 Apr 2021
Ratton TF, Bazzoli N, Santos GB (2003) Reproductive biology of Apareiodon affinis (Pisces: Parodontidae) in the Furnas Reservoir, Minas Gerais. Brazil J Appl Ichthyol 19(6):387–390. https://doi.org/10.1111/j.1439-0426.2003.00485.x
Regan CT (1904) A monograph of the fishes of the family Loricariidae. Trans Linn Soc London 17(3):191–350
Reis RE, Kullander SO, Ferraris Jr. CJ (Orgs.) (2003) Check list of the freshwater fishes of South and Central America. Porto Alegre, Edipucrs, 729p
Retzer ME, Page LM (1996) Systematics of the stick catfishes, Farlowella Eigenmann & Eigenmann (Pisces, Loricariidae). Proc Acad Nat Sci Philadelphia 147:33–88
Roa R, Ernst B, Tapia F (1999) Estimation of size at sexual maturity: an evaluation of analytical and resampling procedures. Fish Bull 97:570–580
Rochet MJ (2000) A comparative approach to life-history strategies and tactics among four orders of teleost fish. ICES J Mar Sci 57:228–239. https://doi.org/10.1006/jmsc.2000.0641
Sabaj MH, Armbruster JW, Page LM (1999) Spawning in Ancistrus (Siluriformes: Loricariidae) with comments on the evolution of snout tentacles as a novel reproductive strategy: larval mimicry. Ichthyol Explor Freshw 10:217–229
Sales CF, Domingos FFT, Brighenti L, Ribeiro RIMA, Santos HB, Thomé RG (2016) Biological variables of Hypostomus francisci (Siluriformes: Loricariidae) from Itapecerica River, Minas Gerais State, Brazil. An Acad Bras Cienc 88:1603–1614. https://doi.org/10.1590/0001-3765201620150513
Santos EP (1978) Dinâmica de populações aplicada à pesca e piscicultura. HUCITEC, Editora da Universidade de São Paulo, São Paulo, p 129p
Secutti S, Trajano E (2009) Reproductive behavior, development and eye regression in the cave armored catfish, Ancistrus cryptophthalmus breed in laboratory. Neotrop Ichthyol 7(3):479–490. https://doi.org/10.1590/S1679-62252009000300016
Suzuki HI, Agostinho AA, Winemiller KO (2000) Relationship between oocyte morphology and reproductive strategy in Loricariid catfishes of the Paraná River, Brazil. J Fish Biol 57:791–807. https://doi.org/10.1111/j.1095-8649.2000.tb00275.x
Taylor JN (1983) Field observations on the reproductive ecology of three species of armored catfishes (Loricariidae: Loricariinae) in Paraguay. Copeia 257–259
Tondato KK, Fialho CB, Súarez YR (2012) Life history traits of Odontostilbe pequira (Steindachner, 1882) in the Pantanal of Porto Murtinho, Mato Grosso do Sul state. Brazil Oecol Aust 16(4):938–950. https://doi.org/10.4257/oeco.2012.1604.11
Tondato KK, Súarez YR, de LA Mateus F, Vicentin W, Fialho CB (2018) Life history characteristics and recruitment of fish under the effect of different hydrological regimes in a tropical floodplain. Environ Biol Fish 101:369–1384. https://doi.org/10.1007/s10641-018-0784-5
Vannote RL, Minshall GW, Cummins KW, Sedell JR, Cushing CE (1980) The river continuum concept. Can J Fish Aquat Sci 37:130–137. https://doi.org/10.1139/f80-017
Vazzoler AEA de M (1996) Biologia da reprodução de peixes teleósteos: teoria e prática. Eduem/SBI/CNPq/Nupelia, Maringá, 169p
Vazzoler AEA de M, Lizama MAP, Inada P (1997) Influências ambientais sobre a sazonalidade reprodutiva. In: Vazzoler AEA de M, Agostinho AA, Hahn NS (eds) A planície de inundação do Alto rio Paraná - Aspectos físicos, biológicos e socioeconômicos. Maringá, Eduem, pp 267–280.
Veloso-Júnior VC, Guimarães-Cruz RJ, Barros MDM, Barata RSL, Santos JE (2009) Reproduction of the lambari Astyanax scabripinnis (Jenyns, 1842) (Pisces: Characidae) in a small stream in Southeastern Brazil. J Appl Ichthyol 25(3):314–320. https://doi.org/10.1111/j.1439-0426.2008.01152.x
Viana D, Wollf LL, Zaleski T, Romão S, Bertoldi G, Donatti L (2008) Population structure and somatic indexes of Hypostomus cf. ancistroides (Siluriformes, Loricariidae) collected from the Bonito River, Ivaí River Basin, Turvo. Paraná Braz Arch Biol Technol 51:493–502. https://doi.org/10.1590/S1516-89132008000300008
Vieira S (1991) Introdução à Bioestatística. Editora do Campus, Rio de Janeiro, p 294
Webb PW (1989) Station-holding by three species of benthic fishes. J Exp Biol 145:303–320
Winemiller KO (1989) Patterns of variation in life history among South American fishes in seasonal environments. Oecologia 81:225–241. https://doi.org/10.1007/BF00379810
Winemiller KO, Rose KA (1992) Patterns of life-history diversification in North American fishes: implications for population regulation. Can J Fish Aquat Sci 49:2196–2218. https://doi.org/10.1139/f92-242
Zardo EL, Behr ER (2015) Population structure and reproductive biology of Loricariichthys melanocheilus Reis & Pereira, 2000 (Siluriformes: Loricariidae) in the rio Ibicuí, Brazil. Neotrop Ichthyol https://doi.org/10.1590/1982-0224-20140052
Zawadzki CH, Pavanelli CS, Langeani F (2008) Neoplecostomus (Teleostei: Loricariidae) from the upper Rio Paraná basin, Brazil with description of three new species. Zootaxa 1757:31–48. https://doi.org/10.11646/zootaxa.1757.1.2
Acknowledgements
We are thankful to our colleagues in the Ichthyology Lab at Universidade Federal do Rio Grande do Sul (UFRGS) for field and laboratory work assistance, and to Marco Azevedo for reviewing an early version of this manuscript and Felipe Zilio for helping with statistics. We also thank the anonymous reviewers whose comments greatly improved this manuscript.
Funding
CAPES (Coordenação de Aperfeiçoamento de Pessoal) provided a PhD scholarship along this study to the first author.
Author information
Authors and Affiliations
Contributions
All authors contributed substantially to this study. Fieldwork was performed by VRL; sample identification and preparation were performed by VRL and KKTC; gonadal developmental stages were analyzed and identified by all authors; data collection and analysis were performed by all authors. The first draft of the manuscript was written by VRL and KKTC, and subsequent text production was performed by all authors. All authors revised previous versions of the manuscript and are in accordance with its final version.
Corresponding author
Ethics declarations
Ethics approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
Consent to participate
All authors agreed with their participation in this manuscript.
Consent for publication
All authors agreed with the publication of this manuscript.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: The author name ‘Karina Keyla Tondato-Carvalho’ was incorrectly written as ‘Karina Keyla Tondato de Carvalho’.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lampert, V.R., Tondato-Carvalho, K.K. & Fialho, C.B. Reproductive traits of two species of suckermouth armored catfishes (Siluriformes: Loricariidae) from a coastal drainage in the southern limits of the Atlantic Forest, Brazil. Environ Biol Fish 105, 885–902 (2022). https://doi.org/10.1007/s10641-022-01295-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10641-022-01295-9