Abstract
Clupeiformes are the most important food fish in the world, and provide a key trophic link in marine food chains. Here we describe broad scale patterns of clupeiform demographic characteristics of the delicate round herring sprat Spratelloides delicatulus on the Great Barrier Reef (GBR). Sampling was conducted over 10° of latitude and two seasons at multiple distances across the GBR shelf. The oldest S. delicatulus sampled was 152 days and the maximum standard length was 74 mm. Age and length maxima increased with latitude conforming with ‘counter gradient theory’ and these patterns were consistent between years. von Bertalanffy relationships showed that growth rates were highest at Northern GBR sites; growth coefficients ranged from 2–6 K year−1, and were lowest on southern reefs, i.e. ‘tropical gradient of growth’. Daily survivorship ranged from 91–97% day−1 at all sites. Hatching dates estimated from counts of daily otolith increments indicated a prolonged spawning season of at least 9 months. Reproductive development indicated a size-based relationship. Males and females matured at similar sizes ranging from 36–38 mm, but fish from southern sites were 30–40 days older. Tropical clupeiforms live fast and die young, and patterns of abundance, composition and demography followed strong environmental gradients which conformed to some existing models.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Temperate clupeiforms such as sardines, menhaden and herring provide some of the most abundant and valuable fisheries in the world (Gulland 1971). While tropical clupeiforms are not as valuable in large-scale fisheries, they sustain important artisanal fisheries along the equatorial Pacific and play an equally important role in the oceanic food chain. Clupeiforms worldwide provide a critical link in food webs, maintaining the connection between the plankton and larger nekton. ‘Bait balls’ of schooling clupeiforms attract commercially important predatory fishes, oceanic birds and fishers alike (Cappo and Kelley 2001). It is well known that mortality rates of early life history stages of marine fishes are extremely high (Bailey and Houde 1989). For tropical clupeiforms, however, a high mortality rate can extend throughout an individual’s life. The available data suggests that tropical baitfish seldom live for more than 5 months and become reproductively viable at an early stage (Milton et al. 1991, 1993).
The Great Barrier Reef spans ~ 2000 km along the east coast of Australia and consists of a diverse range of habitats including thousands of individual coral reefs that extend over 14° of latitude. Tropical fishes of the Order Clupeiformes are common baitfishes associated with reefs. Existing studies on the demography of these fishes are relatively rare and cover either small spatial scales or very broad spatial scales that do not consider environmental variation at spatial scales of less than tens to thousands of kilometres (Milton et al. 1991; Hatakeyama et al. 2005; Meekan et al. 2006; Durieux et al. 2009). Furthermore, tropical age-based demographic studies are few compared to those in temperate regions (Green et al. 2009) and, therefore, form a weak basis for a tropical paradigm.
Patterns of fish growth, and how they vary with latitude, have been described and a number of casual models have been proposed. Inverse relationships between temperature and growth rate (i.e. ‘tropical gradient’ of growth) have been described for reef fishes (Choat and Robertson 2002; Meekan et al. 2003; Robertson et al. 2005) and fish larvae (Houde 1989; Wilson and Meekan 2002). The opposite pattern of growth rate increasing with temperature (i.e. ‘counter gradient’) has been described for other fishes by some authors. For example, contrasting patterns for two species of Sebastes were observed; one of which showed a counter gradient in growth with latitude and the other showed no difference in growth with latitude (Boehlert and Kappenman 1980). Counter gradient trends in growth of Morone saxatalis have been demonstrated experimentally (Conover et al. 1997). Furthermore, the genetic capacity for growth in this study was inversely related to length of the growing season and there was a strong positive correlation with growth and latitude of origin. Longevity may also vary with latitude. In a review, it was concluded that life span varies with temperature in a wide range of ectotherms, and there is also no pattern with latitude if the effect of temperature is removed (Munch and Salinas 2009). A thorough understanding of these types of patterns has taken on new relevance with changing temperature regimes brought about by climate change (Walther et al. 2002; Perry et al. 2005; Munday et al. 2008).
Differences in the growth characteristics of fishes are influenced by temperature, food availability, predator and prey abundance (Jones 1986; Meekan et al. 2003). Strong biological patterns and variation in physical factors both with latitude and across the GBR exist. Potential physical factors of influence include freshwater input, turbidity, temperature, abundance of planktonic food and upwelling (Wolanski 2001; Fabricius et al. 2005; Udy et al. 2005). These factors can influence the demography of a species among mesopopulations within a metapopulation. Abundance and growth of many fishes are known to vary across the GBR shelf (Williams 1982; Williams and Hatcher 1983; Williams et al. 1988; Gust et al. 2001; Kingsford and Hughes 2005; Kingsford et al. 2019), but there are few data on clupeiforms.
It was hypothesized that the demographic characteristics of S. delicatulus would vary across and along the GBR. The broad objective of this study was to compare age, growth, reproductive development and measures of instantaneous daily mortality and survivorship of the delicate round herring sprat (Spratelloides delicatulus) across the GBR and at multiple latitudes (from 14 to 23.8° S).
Materials and methods
Study sites and sampling design
Samples of clupeiforms were collected over 10° of latitude on the GBR during the summer of 2009 to 2010. Additional samples were collected at One Tree Island in winter of 2010 and at Lizard Island in winter of 2011. The focus of winter samples was to determine age max at different times of the year at the two latitudinal extremes of the study area, so only fish > 40 mm SL were aged. In summer of 2009–2010, fish were collected at four latitudinal transects of the GBR (Fig. 1). Water temperatures between L1 and L4 have been documented to differ by about 2 °C in summer and 4 °C in winter (Kingsford et al. 2019). Temperatures measured at L1 to L4 in summers of 2009 to 2010 using a CTD ranged between 29.4 and 26.4 °C and the water columns were well mixed (10–20 m deep). Additional samples for histological gonad analysis were collected from the Capricorn Bunker and Lizard Island latitudes in summer of 2014–2015.
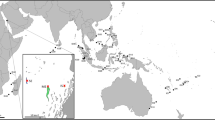
Map of the four latitudinal transects off the Queensland coast, Great Barrier Reef, Australia. Containing 15 sites distributed by strata inner, mid, and outer from the mainland (an example of distance strata is provided in the inset). Specific sample sites at each latitude listed in Table 1
In the summer of 2009 to 2010 and within each latitudinal transect, samples were collected near reefs at inner, mid and outer shelf strata (except at the Capricorn Bunker latitude where only outer shelf sites were present). Care was taken to ensure these traps were separated by sufficient distance (> 500 m) to maintain independence. Where possible, two sites within a cross shelf distance were sampled, and this occurred at the mid-shelf distance for the Lizard transect and the mid-shelf distance of the Cairns transect; n = 2 replicate traps per site (sites see Table 1). Fish were captured using light traps (12 V, 8 W Fluoro tube), which fished at a depth of approximately 1.5 to 2 m, in water columns of 10–15 m deep at each site. Collections of fish for measurements of size and age were from traps that fished for 3 to 4 h, depending on ease of pick up. This time window of collection was consistent at all reefs.
Samples of fish were preserved immediately upon capture in 80% ethanol to prevent dissolution of otoliths. Voucher specimens were also sorted from traps to be stored in 10% formalin for later identification. Because it was possible that light traps would not sample largest fish, additional fish samples were collected at night from the boat for age-based analyses, using 1000 W underwater lights and hand nets where possible.
Validation of daily rings is notoriously difficult for these fishes. Previous studies have validated daily rings for a total of four individual Spratelloides gracilis and 15 S. robustus over a maximum of 29 days (Milton et al. 1991; Rogers et al. 2003). In an experiment to validate daily sagittal increments, S. delicatulus caught in light traps at One Tree Island Research Station were placed in a 10-L aerated container with 0.25 g L−1 tetracycline-treated seawater for 12 h. Surviving fish were then removed from the treatment and transferred to a 1000-L holding tank containing seawater. The fish were fed wild zooplankton caught daily with a 100-µm mesh net and were sacrificed after either 3 or 10 days in the tank and stored in ethanol.
Otolith processing
Sagittal otoliths were dissected from each fish and stored in Eppendorf tubes. A sagittal otolith from each fish was then embedded in Crystal Bond (a thermoplastic glue) with the primordium located close to the edge of the slide. The otolith was ground using 3 µm lapping film on a grinding wheel down to the edge of the slide. The half otolith was then up-righted and fixed to the centre of another glass slide, and polished down to leave a thin transverse section containing the primordium (20–40 µm thick). Increments were counted under a compound microscope at 400 × to 1000 × with transmitted light and immersion oil. Digital images of otoliths were taken and processed using the Leica Application Suite (LAS), or counts were made directly while viewing under the microscope. The sagittae of a small number of fish that were less than 20 mm SL were mounted and read whole.
Counts were made from the first clear increment, closest to the primordium, and outwards along the longest (ventral) axis of the otolith section. Counts of increments were made separately without knowledge of fish length and slides were examined in random order. Each otolith was counted twice with a minimum of 48 h between each count. The quality control criteria for age data were if sequential reads revealed a > 5% age error then a third count was conducted. Any otoliths with continuing age discrepancy among reads were removed from further analysis. This method is accepted among fish biologists to give the best estimated of age (Cope and Punt 2007). A total of 1095 fish from multiple sites across the GBR were aged and included in age analyses.
Reproductive analysis
Samples of S. delicatulus were collected for histological analysis of reproductive development at two latitudes separated by over 1000 km in the Austral summer of 2014–2015. Within each latitude, three outer reefs were sampled using light traps as per the previous sampling. Once collected, individual fish were selected at random, measured in length (SL) and transversally cut with the posterior two-thirds preserved in 10% formalin to preserve the gonads. Two hundred samples were stained with hematoxylin and eosin using standard techniques. A minimum of 10 longitudinal sections per fish were examined under a light microscope and the relative percentage area of each stage of gametogenesis was used to define the reproductive stage, according to Milton and Blaber (1991) and Hatakeyama et al. (2005). Mature fish were defined as stage III or greater, with stages I and II individuals pooled as ‘immature’ and sexed where possible. A minimum of 30 ova were required for analysis in females while males required the presence of at least 100 μm2 of male spermatogenic tissue. Data were based on 16 indeterminate fish, 25 immature females, 33 immature males, 63 mature males and 62 mature females, with a total of 100 fish from each of the Lizard (L1) and Capricorn Bunker (L4) regions. The distribution of lengths in samples from the Lizard and Capricorn Bunker regions was similar with means (± SE) of 42 ± 0.6 mm and 41 ± 0.7 mm, respectively. Sex ratios of differentiated individuals within each latitude were tested for differences from a 1:1 ratio of males to females using a two-tailed, two sample Z-tests of proportions. A single sample Z-test was used on individuals from both latitudes to test overall if there was a difference in male to female ratios. Comparisons of maturity and size were performed by fitting binomial logistic models in R using the package ‘glm’ with the following factors: standard length, region and sex. Only individuals which were able to be differentiated were used in this analysis and individuals classified as mature were coded as 1 and immature coded as 0 (Ruiz-Abierno et al. 2021). The data were checked for assumptions by q-q plots, dispersion and outlier tests in the package ‘DHARMa’ from 250 simulations. The data were then fitted using a logit link function (Table S2, Eq. 1), which scored a better goodness of fit score (AIC score) than the alternative probit and complimentary log–log link functions when validating the model. The model used was selected by stepwise deletion of factors from the maximal model (Boulcott and Wright 2008). To determine the effect a change of a single unit in a parameter had on the probability of a fish being mature, the coefficients were back transformed by calculating their exponential as an odds ratio. A quasi-R2 was calculated as per Table S2 Eq. 2 and the size at which an individual had a 50% chance of being mature (L50) calculated as per Table S2 Eq. 3, with the procedures adapted from Chen and Paloheimo (1994), Boulcott and Wright (2008) and Hoffmann et al. (2017).
Analysis of growth age was estimated from counts of daily increments in sagittal otoliths of S. delicatulus. To describe the relationship between age and length over the complete size range of fish sampled, a constrained form of the von Bertalanffy growth curve was fitted using the formula:
where L(t) = length of fish at age t.
L∞ = asymptotic length.
K = growth co-efficient.
t0 = age at which L = (0), constrained to known length of S. delicatulus at hatching (von Bertalanffy 1938).
The constrained version of this equation accounts for fish length at time of hatching (t0) as L(0) = 4.3 mm (Leis and Carson-Ewart 2000). Other studies have used a re-parameterized form of the von Bertalanffy equation, to ensure no extrapolation of L∞ past L (max). All sites contained large fish, so it was assumed that these samples were adequate representations of the maximum size classes for S. delicatulus and a standard, constrained von Bertalanffy equation was fitted. It should be noted that the results from our study are comparable with the re-parameterized von Bertalanffy values given in the literature for other Spratelloides species from different regions (Milton et al. 1991, 1993; Rogers et al. 2003; Meekan et al. 2006). Comparisons of K and L∞ were done among latitudes using regression with latitude as the independent variable. Residuals were checked for all regressions to meet the assumptions that the residuals were randomly distributed. Length at age data analysed using the von Bertalanffy model were also compared with the Gompertz model. Although the Gompertz equations often resulted in a little less variation (Table S1), we have presented the von Bertalanffy equations for comparisons with other studies. Growth models were done in EXCEL and multiple iterations were run using Solver to vary parameters of the models with the objective of minimising variances.
Calculations of mortality
The total instantaneous rate of mortality (Z) was calculated using loge-linear regression analyses of age-frequency data of S. delicatulus; equal recruitment among years was assumed. Age classes to the left of the age-frequency distribution maxima were excluded from the analysis to calculate the rate of the negative slope only. These data are deleted as they are not independent of sampling method bias (Hilborn and Walters 1992). The slope of the regression line between age classes was used to estimate the instantaneous mortality rate (Z) following the equation:
where F = fishing mortality rate and M = natural mortality rate (Hilborn and Walters 1992; Kingsford and Hughes 2005). Since there is no commercial or recreational fishery for Spratelloides on the GBR, F equals zero and therefore Z is equivalent to M. From the regression equations, daily mortality rates could be calculated.
Tests for differences in instantaneous mortality were made between distances at each latitude (n = 3 distances); sites were pooled within a distance. Furthermore, because mortality rates were similar within a latitude, instantaneous mortality rates were compared among all latitudes (n = 4 latitudes), using individual values of Z from within latitudes.
Daily survivorship rate (S) was calculated according to Formula (3) as expressed as percentage survival per day (see Hilborn and Walters 1992).
Calculations based on total instantaneous rate of mortality (Z) assume equal recruitment for each age cohort, but this is of course questionable (Hilborn and Walters 1992). Our intent, therefore, was to provide multiple estimates of Z and S at different spatial scales to get a broad overview of estimates of mortality and related survivorship.
Results
Age frequency and maxima
A short-term otolith marking experiment revealed that sectioned otoliths from fish treated with tetracycline had a fluorescent marker close to the otolith edge (Suppl. Figure 1). Only eight fish survived the treatment (89% fish mortality). There was close agreement between the number of increments laid down after the treatment and the number of days fish were held after treatment. Fish that were sacrificed after 3 days of treatment had a mean increment number of 2.8 (n = 5, range = 2–3). Those otoliths removed after 10 days had a mean increment number of 9.3 days (n = 3, range = 9–10). Along with two other studies, it was concluded that increments were deposited daily (Milton et al. 1991; Rogers et al. 2003).
The age of S. delicatulus collected in this study ranged from 9 to 152 days; the oldest fish was collected at One Tree Island in winter of 2011. The age maximum of fish increased with latitude (Table 2) and there was a positive relationship between age maximum and length maximum at all sites (regression ANOVA df(1,11), y = 2.521x – 35.627, r2 = 0.66 s, P = 0.0004). Furthermore, the oldest 5% of S. delicatulus increased with latitude (Table 2). Some S. delicatulus from the Southern GBR lived for at least a month longer than fish in the Northern GBR. Spratelloides delicatulus were consistently below 82 days old and 50 mm at all shelf strata at Lizard Island (L1), whereas a greater range of ages were found from Cairns and further south (L2 to L4, Fig. 2, Table 2). There was little evidence that age frequency varied cross shelf (Fig. 2). The age distribution was largely uni-modal at Lizard Island sites (i.e. L1, inner to outer shelf), and multi-modal at other sites indicating multiple age-cohorts of recruits (e.g. Orpheus and Britomart; Fig. 2).
Age frequency distributions of S. delicatulus collected from sites at three distance strata and four latitudes in the summer of 2009 to 2010 (bin size 2 days), note that no fish were caught at the inner L2 Cairns site and that inner and mid-shelf reefs are absent in the Capricorn Bunker. NR, no reef; NF, no fish caught
Spawning took place in multiple of months of the year. When the birthdate of fish was back-calculated, our collections from December to February included birthdates from September to January while fish collected at Lizard Island in June 2011 had birthdates from March to April. Fish collected at One Tree Island in October 2010 had birthdates in July.
Growth
There were great differences among latitudes in the parameters K, L∞ and t0 and the constrained von Bertalanffy growth curves (Fig. 3a-b, Fig. 4; Table 3). Contrasting patterns were found for the parameters of growth (L∞, K). K decreased with an increase in latitude while L∞ increased with latitude. Accordingly, a significant inverse relationship was found between the growth rate (K) and L∞ for S. delicatulus (regression and ANOVA, df(1,9), y = − 0.0002x + 0.0246, r2 = 0.90, P = 0.000005. A comparison of age at 40–50 mm provided further evidence that the rate of growth to L∞ varied with latitude (Fig. 3c). Age at 40–50 mm SL increased with latitude as would be expected with lower values of K. Furthermore, these findings concurred with fish collected in two seasons. The 40–50 mm SL size class of fish at all outer sites was compared among latitudes, and as for data pooled by latitude, a strong north–south pattern was detected where age at size increased with latitude; differences among latitude were significant (ANOVA df(1,3), F = 116.5, P = 0.001701; Fig. 3c). Patterns cross shelf were minor compared to differences in K and L∞ among latitudes (Table 3).
Relationship between von Bertalanffy parameters for S. delicatulus a) K year−1 (growth coefficient) pooled by latitude, b) L∞ (mean asymptotic length mm) by latitude, and c) the average age of fish, 40–50 mm SL, by latitude. Lizard and Capricorn Bunker latitudes show replicate age data from summer and winter (Table 1)
von Bertalanffy growth curves for S. delicatulus collected from eleven sites within distance and latitude strata (North on the right and South on the left) during the summer of 2009–2010, note that no fish were caught at the inner L2 Cairns site and that inner and mid-shelf reefs are absent in the Capricorn Bunker
Reproductive development
Of the 200 S. delicatulus analysed, the smallest was 24 mm standard length and the largest 55 mm. Sex differentiation was apparent in individuals as small as 24 mm with no undifferentiated individuals observed larger than 36 mm. Immature males were detected at up to 48 mm in the Capricorn Bunker (L4) but only 42 mm in the Lizard Region (L1). Immature females were observed up to 39 mm at both latitudes. The sex ratios for differentiated individuals were not significantly different to a 1:1 ratio in either latitude (Z Ratio Test, df = 2, Z = 0.567, P = 0.753) nor for differentiated individuals pooled across latitudes (Z Ratio Test, df = 1, Z = 0.440, P = 0.507).
Mature males and females were detected at Lizard from 36–37 mm SL, while in the Capricorn Bunker, mature fish were detected from 38 mm SL. The binomial logistic regression performed found that standard length and latitude (Lat) were both significant factors in the maturity schedules of S. delicatulus (Table 3S). The L50 for samples from the Capricorn Bunker (L4) was calculated at 40.1 mm SL, while the L50 estimated for the Lizard (L1) was 38.3 mm SL (Fig. 5). Although this difference was less than 2 mm, it was statistically significant and fishes in the size range 38–40 mm SL would mature at 30 to 40 days older at L4 when compared with L1 (Fig. 4).
Mortality
Spratelloides delicatulus had very high rates of daily mortality at all latitudes and distances and sites within latitudes in the summer of 2009 to 2010 (Table 4, Fig. 6). Instantaneous mortality rate Z for S. delicatulus ranged from 0.028 to 0.097 among sites on the GBR and daily rates of survivorship varied from 90.8 to 97.3% (Table 4). The lowest survival rate was 90.8% day−1 at a northern site.
The highest estimates of daily mortality, based on pooled Z, were found at the Lizard transect on the Northern GBR (2.5% day−1) and lowest in the Southern GBR (1.4% day−1). Pooled daily survival (S%) by latitude was about 1% less at low latitudes (Table 4).
Discussion
Spratelloides delicatulus are very short-lived fish with fast population turnover rates, and conformed to a ‘tropical gradient’ for growth (K) to L∞, and a ‘counter gradient’ pattern for L∞ and age max both of which increased with latitude. No fish sampled in this study survived beyond 152 days and size maximum was similar among latitudes (51 to 74 mm SL). Milton et al. (1991) found a similar age maximum for S. delicatulus in the Central Pacific to what we found in the Northern GBR. In our study, age maxima was 2 to 3 months less in the warmer waters of the Northern GBR which conforms with the counter gradient patterns for age max. Repeated sampling in the winters of 2010 and 2011 revealed similar differences in age maximum at both the Lizard and Capricorn Bunker latitudes, thus providing evidence that time of sampling was not confounding the latitudinal patterns found. It should be noted that the fish were collected at or close to the maximum recorded size of 70 mm SL on FishBase. Furthermore, this absolute size maximum was rarely collected in our study or by others (Whitehead et al. 1988; Froese and Pauly 2020). This was reassuring as by site fish rarely reached the asymptotic length (L∞) of the von Bertalanffy equation. Although fish from the most northern and southern sites matured at a similar size, fish from lowest latitudes matured more than a month earlier than those from the highest latitudes.
The shortest lived vertebrate is the goby Eviota sigillata (Depczynski and Bellwood 2005) at 59 days old, while orange roughy (Hoplostethus atlanticus) may live to 149 years (Fenton et al. 1991). Clearly the age maximum of Spratelloides (152 days) is close to the minimum age maximum for vertebrates. A back calculation from age frequency data revealed that spawning takes place over an extended season. Given we found birthdays in winter, spring and summer, it is highly likely that they spawn all year which has been previously suggested (Dalzell 1993) and aligns with collections of large numbers of Spratelloides larvae in summer (January/February) and winter at One Tree Island in June–July (i.e. Austral Winter; Kingsford 2001)). However, more detailed temporal sampling would be required to demonstrate seasonality and potential periodicity within such as lunar-related patterns (Johannes 1978). Such a short-lived organism experiences a biological limitation in life, and a short window in which it can contribute to the next generation (Depczynski and Bellwood 2005). A result of the short life, high mortality and high population turnover rate of Spratelloides implies that population bottlenecks would be frequent, and periods of high larval abundance with few mature fish are likely to be common within and among reefs at different latitudes. Tropical Spratelloides have the highest growth parameters of all clupeiforms recorded in the literature. Temperate and sub-tropical species reach 0.5 to 1 K year−1 (Ahrenholz 1991; Emmett et al. 2005), while S. delicatulus reaches up to 6 K year−1 on the GBR (this study).
Fish growth generally varies along latitudinal gradients and two main patterns have been observed as follows: a ‘tropical gradient’ (an increase in growth with a decrease in latitude) and the opposite ‘counter gradients’, though there are exceptions where some taxa show no gradient in age max or growth with latitude (e.g. some Sebastes, Boehlert and Kappenman 1980). Tropical gradient models state that growth is slower in cold water (Houde 1989; Atkinson and Sibly 1997), while the counter gradient models propose that growth is slower in warm water (Yamahira and Conover 2002). These theories are principally driven by a gradient in water temperature which has been clearly demonstrated in our study and by others in the over the same latitudinal range (2–4 °C, Kingsford et al. 2019). This can explain why fish maximum length can be the same across multiple latitudes, yet growth rates differ significantly. Our results for the growth of tropical Spratelloides conform to a tropical gradient model as suggested by Houde (1989) and Atkinson and Sibly (1997).
Differences in growth curve trajectories between geographic regions may be determined by both environmental and genetic influences (Sebens 1987). Key factors driving the differences in growth are predation pressure, temperature, effects on metabolism, resource variation (abundance of plankton) and variable water condition (turbidity). Wave exposure, sediment, depth and topographical complexity are all known to correlate with growth patterns; however, these factors are considered to be relatively consistent across all latitudes for a given distance for the mainland (Wenger et al 2012; Kingsford et al 2019). Genetic differences among latitudes are possible, but not described. Temperature gradient is likely to be the key driver of differences in growth patterns. This difference is known to fluctuate seasonally and is generally greater during the winter months with a disproportionate drop at higher latitudes (Wolanski 2001).
Density dependence and resource limitation is thought to be more intense in low latitudes (Atkinson and Sibly 1997; Gust et al. 2002) but there were few data on density effects and resource abundance in small planktivorous fishes. We predict that higher levels of competition with other Spratelloides species such as S. gracilis and S. lewisi could occur at lower latitudes but there are few data on abundance. However, we suggest that the importance of these effects is likely to be highly site dependent and have little influence on latitudinal patterns.
An extended spawning and growing season exists at lower latitudes with higher temperatures (Cushing 1975). In the tropics, species generally experience a narrower range of seasonal variation in sea temperature than temperate species (Munday et al. 2008). Although most of the GBR is contained within the tropics, there is still a clear gradient in seasonality and variation in water temperatures over 10° of latitude; this in turn can be related to latitudinal growth theories. Most theory is based on temperate to cold-temperate environments where it is argued that fishes at low latitudes generally experience a longer growing season with short pulses of high productivity and peaks in hatching (Cushing 1975). In contrast, at high latitudes, fishes have shorter growing seasons with, on average, consistently high productivity and continuous hatching (Fiedler et al. 1991). Therefore, fish potentially maximize their use of this short period resource at high latitudes. In contrast, the productivity cycle at low latitudes is less extreme and this resource is spread out more evenly throughout the season (Conover 1992). However, cold water is also known to slow metabolic and growth processes (Schmidt-Nielsen 1997). The direct applicability of these assumptions to tropical to subtropical latitudes is yet to be tested. However, lower mean temperatures and shorter growing seasons at higher latitudes may cause a reduction in the annual growth rate of an individual, as demonstrated for S. delicatulus in this study.
Life history characteristics are a result of this trade-off between survival and reproduction (Stearns 1976). Fish with faster life history, such as clupeiforms (early maturity, high growth rate, small body size), allocate more resources to reproductive output than those with slower life histories (Denney et al. 2002), resulting in extremely high rates of mortality. Mortality rates were high across all latitudes and sites of this study. Pooled daily mortality rate ranged from 0.9 to 2.5% day−1 and site-specific mortality ranged from 9.2 to 2.7% day−1. Previous work showed that S. gracilis had a similar high daily mortality rate of approximately 3.7% at on the North West Shelf of Western Australia (Meekan et al. 2006).
A fish reproductive strategy may shift in relation to environmental conditions, so breeding patterns are a conditional strategy (McBride et al. 2015). Reproductive development is fast with sexual differentiation observed from 24 mm and maturity beginning at 36–38 mm across the two latitudes sampled. These values are similar to those observed in previous studies which have also indicated the ability for S. delicatulus to repeat spawn (Milton et al. 1991, 1994). The shorter time frame available to fishes at low latitudes as well as the onset of maturity a month earlier than higher latitude individuals suggest for S. delicatulus in a ‘tropical gradient of growth’ a higher reproductive allocation of energy at low latitudes, perhaps resulting in a shorter lifespan. The differences in growth and age maxima could also reflect genetic differences at large spatial scales. We cannot exclude the possibility for genetic variation driving differences in the North and South GBR populations.
In conclusion, there were clear differences in age maxima and growth characteristics for populations of S. delicatulus along the GBR. This species had a short lifespan of age-max 48–152 days with fast growth. Furthermore, age maxima were up to 98 days greater in high latitudes and concurred with a ‘counter gradient’ model. In contrast, growth to L∞ was highest at low latitude and matched a ‘tropical gradient of growth’. Although fish matured at a similar size regardless of sex or latitude, they matured 30–40 days earlier at the lowest latitudes. Mortality was very high among all latitudes. The North–South axis of the GBR provides an excellent opportunity to test growth theory and our data provide a baseline for potential demographic variation with environmental change.
Data availability
Available upon reasonable request to the authors.
Code availability
The software SYSTAT Ver 13 is publicly available, (https://www.statcon.de/shop/en/software/statistics/systat) and was used for most graphics. R version 4.1.2 was used to test the maturity schedules of males and females between latitudes using the packages: tidyr, dplyr, DHARMa, effects, multcomp, emmeans, outliers, ggplot2 and ggiraph; the code used is available on request. Growth models were done in EXCEL (Ver 2102; Build # 13,801.21004) and multiple iterations were run using Solver to vary parameters of the models with the objective of minimising variances.
References
Ahrenholz DW (1991) Population biology and life history of the North American menhadens, Brevoortia spp. Mar Fish Rev 53:3–19
Atkinson D, Sibly RM (1997) Why are organisms usually bigger in colder environments? Making sense of a life history puzzle. Trends Ecol Evol 12:235–239
Bagenal TB, Tesch FW (1978) Methods for assessment of fish production in fresh waters. In: Bagenal TB (ed) Age and growth. Blackwell Scientific Publications, Oxford, pp 101–136
Bailey KM, Houde ED (1989) Predation on eggs and larvae of marine fishes and the recruitment problem. Adv Mar Biol 25:1–83
Boehlert G, Kappenman R (1980) Variation of growth with latitude in two species of rockfish (Sebastes pinniger and S. diploproa) from the Northeast Pacific Ocean. Mar Ecol Prog Ser 3:1–10
Boulcott P, Wright PJ (2008) Critical timing for reproductive allocation in a capital breeder: evidence from sandeels. Aquat Biol 3:31–40
Cappo M, Kelley R (2001) Connectivity in the Great Barrier Reef World Heritage Area: an overview of pathways and processes. In: Wolanski E (ed) Oceanographic Processes of Coral Reefs: Physical and Biological Links in the Great Barrier Reef. CRC Press, Boca Raton, pp 161–187
Chen Y, Paloheimo JE (1994) Estimating fish length and age at 50% maturity using a logistic type model. Aquat Sci 56:206–219. https://doi.org/10.1007/BF00879965
Choat JH, Robertson DR (2002) Age-based studies. In: Sale PF (ed) Coral reef fishes: dynamics and diversity in a complex ecosystem. Academic Press, San Diego, pp 57–80
Conover DO (1992) Seasonality and the scheduling of life history at different latitudes. J Fish Biol 41:161–178
Conover DO, Brown JJ, Ehtisham A (1997) Countergradient variation in growth of young striped bass (Morone saxatilis) from different latitudes. Can J Fish Aquat Sci 54:2401–2409
Cope JM, Punt AE (2007) Admitting ageing error when fitting growth curves: an example using the von Bertalanffy growth function with random effects. Can J Fish Aquat Sci 64:205–218
Cushing DH (1975) Marine ecology and fisheries. Cambridge University Press, Cambridge
Dalzell P (1993) Small pelagic fishes. In: Wright A, Hill L (eds) Nearshore marine resources of the South Pacific: information for fisheries development and management. Institute of Pacific Studies; Forum Fisheries Agency; International Centre for Ocean Development, Suva; Honiara; Canada, pp 97–133
Denney NH, Jennings S, Reynolds JD (2002) Life-history correlates of maximum population growth rates in marine fishes. Proc R Soc B Biol Sci 269:2229–2237
Depczynski M, Bellwood DR (2005) Wave energy and spatial variability in community structure of small cryptic coral reef fishes. Mar Ecol Prog Ser 303:283–293
Durieux EDH, Meekan MG, Ponton D, Vigliola L (2009) Temperature, selective mortality and early growth in the short-lived clupeid Spratelloides gracilis. J Fish Biol 74:921–938
Emmett RL, Brodeur RD, Miller TW et al (2005) Pacific sardine (Sardinops sagax) abundance, distribution, and ecological relationships in the Pacific Northwest. Calif Coop Ocean Fish Investig Reports 46:122–143
Fabricius K, De’ath G, McCook L et al (2005) Changes in algal, coral and fish assemblages along water quality gradients on the inshore Great Barrier Reef. Mar Pollut Bull 51:384–398. https://doi.org/10.1016/j.marpolbul.2004.10.041
Fenton GE, Short SA, Ritz DA (1991) Age determination of orange roughy, Hoplostethus atlanticus (Pisces: Trachichthyidae) using210Pb:226Ra disequilibria. Mar Biol 109:197–202
Fiedler PC, Philbrick V, Chavez FP (1991) Oceanic upwelling and productivity in the eastern tropical Pacific. Limnol Oceanogr 36:1834–1850
Froese R, Pauly D (2020) www.fishbase.org. In: World Wide Wedb Electron. Publ. www.fishbase.org. Accessed 16 Jul 2020
Green BS, Mapstone BD, Carlos G, Begg GA (2009) Tropical fish otoliths: information for the assessment, management and ecology. Springer Verlag, New York
Gulland JA (1971) Ecological aspects of fishery research. In: Cragg JB (ed) Advances in Ecological Research, 7th edn. Academic Press, London, pp 115–176
Gust N, Choat JH, Ackerman JL (2002) Demographic plasticity in tropical reef fishes. Mar Biol 140:1039–1051
Gust N, Choat JH, McCormick MI (2001) Spatial variability in reef fish distribution, abundance, size and biomass: a multi-scale analysis. Mar Ecol Prog Ser 214:237–251
Hatakeyama R, Shirafuji N, Nishimura D et al (2005) Gonadal development in early life stages of Spratelloides gracilis. Fish Sci 71:1201–1208
Hilborn R, Walters CJ (1992) Quantitative fisheries stock assessment; choice, dynamics and uncertainty. Chapman and Hall, New York
Hoffmann GCS, Freitas MO, Moura RL et al (2017) Reproductive biology of Haemulon plumierii in the south-western Atlantic Ocean’s most extensive reefs: implications for fisheries management. J Fish Biol 90:2111–2124
Houde ED (1989) Comparative growth, mortality, and energetics of marine fish larvae: temperature and implied latitudinal effects. Fish Bull 87:471–495
Johannes RE (1978) Reproductive strategies of coastal marine fishes in the tropics. Environ Biol Fishes 3:65–84
Jones GP (1986) Food availability affects growth in a coral reef fish. Oecologia 70:136–139
Kingsford M, Hughes J (2005) Patterns of growth, mortality, and size of the tropical damselfish Acanthochromis polyacanthus across the continental shelf of the Great Barrier Reef. Fish Bull 103:561–573
Kingsford MJ (2001) Diel patterns of abundance of presettlement reef fishes and pelagic larvae on a coral reef. Mar Biol 138:853–867
Kingsford MJ, Welch DJ, O’Callaghan M (2019) Latitudinal and cross-shelf patterns of size, age, growth, and mortality of a tropical damselfish Acanthochromis polyacanthus on the Great Barrier Reef. Diversity 11:1–19
Leis JM, Carson-Ewart M (2000) The Larvae of Indo-Pacific Coastal Fishes. In: Second Edition: An Identification Guide to Marine Fish Larvae (Fauna Malesiana Handbooks Fauna Malesiana Handbooks), 2nd edn. Brill, Leiden, pp 850
McBride RS, Somarakis S, Fitzhugh GR et al (2015) Energy acquisition and allocation to egg production in relation to fish reproductive strategies. Fish Fish 16:23–57
Meekan MG, Carleton JH, McKinnon AD et al (2003) What determines the growth of tropical reef fish larvae in the plankton: Food or temperature? Mar Ecol Prog Ser 256:193–204
Meekan MG, Vigliola L, Hansen A et al (2006) Bigger is better: size-selective mortality throughout the life history of a fast-growing clupeid, Spratelloides gracilis. Mar Ecol Prog Ser 317:237–244
Milton DA, Blaber SJM (1991) Maturation, spawning seasonality, and proximate spawning stimuli of six species of tuna baitfish in the Solomon Islands. Fish Bull 89:221–237
Milton DA, Blaber SJM, Rawlinson NJF (1993) Age and growth of three species of clupeids from Kiribati, tropical central south Pacific. J Fish Biol 43:89–108
Milton DA, Blaber SJM, Rawlinson NJF (1991) Age and growth of three species of tuna baitfish (genus: Spratelloides) in the tropical Indo-Pacific. J Fish Biol 39:849–866
Milton DA, Blaber SJM, Rawlinson NJF (1994) Reproductive biology and egg production of three species of Clupeidae from Kiribati, tropical central Pacific. Fish Bull 92:102–121
Munch SB, Salinas S (2009) Latitudinal variation in lifespan within species is explained by the metabolic theory of ecology. PNAS 106:13860–13864
Munday PL, Kingsford MJ, O’Callaghan M, Donelson JM (2008) Elevated temperature restricts growth potential of the coral reef fish Acanthochromis polyacanthus. Coral Reefs 27:927–931
Perry AL, Low PJ, Ellis JR (1912) Reynolds JD (2005) Ecology: Climate change and distribution shifts in marine fishes. Science 308(80):1915
Robertson DR, Choat JH, Posada JM et al (2005) Ocean surgeonfish Acanthurus bahianus. II. Fishing effects on longevity, size and abundance? Mar Ecol Prog Ser 295:245–256
Rogers PJ, Geddes M, Ward TM (2003) Blue sprat Spratelloides robustus (Clupeidae: Dussumieriinae): a temperate clupeoid with a tropical life history strategy? Mar Biol 142:809–824
Ruiz-Abierno A, Márquez-Fariás JF, Trápaga-Roig M, Hueter RE (2021) Length at maturity of two pelagic sharks (Isurus paucus and Carcharhinus longimanus) found off northern Cuba. Bull Mar Sci 97:77–88
Schmidt-Nielsen K (1997) Animal physiology: adaptation and environment, 5th edn. Cambridge University Press, Cambridge
Sebens KP (1987) The ecology of indeterminate growth in animals. Annu Rev Ecol Syst 18:371–407
Stearns SC (1976) Life history characteristics: a review of the ideas. Q Rev Biol 51:3–47
Systat (2009) SYSTAT ver. 13 for Windows
Udy J, Gall M, Longstaff B et al (2005) Water quality monitoring: a combined approach to investigate gradients of change in the Great Barrier Reef, Australia. Mar Pollut Bull 51:224–238
von Bertalanffy L (1938) A quantitative theory of organic growth (inquiries on growth laws II). Hum Biol 10:181–213
Walther GR, Post E, Convey P et al (2002) Ecological responses to recent climate change. Nature 416:389–395
Wenger AS, Johansen JL, Jones GP (2012) Increasing suspended sediment reduces foraging, growth and condition of a planktivorous damselfish. J Exp Mar Biol Ecol 428:43–48
Whitehead PJP, Nelson GJ, Wongratana T (1988) FAO Species Catalogue. Clupeoid fishes of the world. In: An annotated and illustrated catalogue of the herrings, sardines, pilchards, sprats, shads, anchovies and wolf-herrings, vol 7. Part 1 - Chirocentridae, Clupeidae and Pristigasteridae. FAO Fisheries Synopsis, Rome, pp 305–579
Williams DM, Hatcher A (1983) Structure of fish communities on outer slopes of inshore, mid-shelf and outer shelf reefs of the Great Barrier Reef. Mar Ecol Prog Ser 10:239–250
Williams DMB (1982) Patterns in the distribution of fish communities across the Central Great Barrier Reef. Coral Reefs 1:35–43
Williams DMB, Dixon P, English S (1988) Cross-shelf distribution of copepods and fish larvae across the central Great Barrier Reef. Mar Biol 99:577–589
Wilson DT, Meekan MG (2002) Growth-related advantages for survival to the point of replenishment in the coral reef fish Stegastes partitus (Pomacentridae). Mar Ecol Prog Ser 231:247–260
Wolanski E (2001) Physical and biological links in the Great Barrier Reef. CRC Press, Australia
Yamahira K, Conover DO (2002) Intra- vs interspecific latitudinal variation in growth: adaptation to temperature or seasonality. Ecology 83:1252–1262
Acknowledgements
We would like to thank Mark O’Callaghan, Emily Gerard of the Reef and Ocean Ecology Laboratory, JCU, and Kari Arbouin and all other volunteers for field and lab assistance during this study. We also thank the One Tree Island Research Station for field assistance and support, and the crews from the following charter vessels: Phoenix of Bianca Charters, Kalinda and Melantre. Funding for this project was awarded to MJK from the ARC Centre of Excellence of Coral Reef Studies and Marine and Tropical Sciences Research Facility (MTSRF).
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions This research was supported by funding to MJK from the ARC Center of Excellence for Coral Reef Studies.
Author information
Authors and Affiliations
Contributions
All of the authors were involved in the collection of fish, processing of data, and drafts of the paper. EW aged the fish and KH-B assessed the reproductive status of fish.
Corresponding author
Ethics declarations
Ethics approval
Animal Ethics: permit for samples collected for aging fish A1487 and GBRMPA Permit numbers G06/202 34.1 and G10/332 39.1. Animal Ethics for reproductive samples (A1808) and GBRMPA permit number G13/35909.1.
Consent to participate
NA.
Consent for publication
The three authors agree that the paper is original and should be published.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visithttp://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kingsford, M.J., Hartog-Burnett, K. & Woodcock, E.J. Demographic patterns of the tropical baitfish Spratelloides delicatulus (Order: Clupeiformes) across the Great Barrier Reef shelf and at multiple latitudes. Environ Biol Fish 105, 461–476 (2022). https://doi.org/10.1007/s10641-022-01246-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10641-022-01246-4