Abstract
Habitat use can be complex, as tradeoffs among physiology, resource abundance, and predator avoidance affect the suitability of different environments for different species. Green sturgeon (Acipenser medirostris), an imperiled species along the west coast of North America, undertake extensive coastal migrations and occupy estuaries during the summer and early fall. Warm water and abundant prey in estuaries may afford a growth opportunity. We applied a bioenergetics model to investigate how variation in estuarine temperature, spawning frequency, and duration of estuarine residence affect consumption and growth potential for individual green sturgeon. We assumed that green sturgeon achieve observed annual growth by feeding solely in conditions represented by Willapa Bay, Washington, an estuary annually frequented by green sturgeon and containing extensive tidal flats that harbor a major prey source (burrowing shrimp, Neotrypaea californiensis). Modeled consumption rates increased little with reproductive investment (<0.4%), but responded strongly (10–50%) to water temperature and duration of residence, as higher temperatures and longer residence required greater consumption to achieve equivalent growth. Accordingly, although green sturgeon occupy Willapa Bay from May through September, acoustically-tagged individuals are observed over much shorter durations (34 d + 41 d SD, N = 89). Simulations of <34 d estuarine residence required unrealistically high consumption rates to achieve observed growth, whereas longer durations required sustained feeding, and therefore higher total intake, to compensate for prolonged exposure to warm temperatures. Model results provide a range of per capita consumption rates by green sturgeon feeding in estuaries to inform management decisions regarding resource and habitat protection for this protected species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fishes move among different environments to improve their rates of survival, growth, and reproduction, ultimately increasing fitness (Clark and Levy 1988; Armstrong et al. 2013; Glover et al. 2013; Manderson et al. 2014). Because most fish cannot physiologically thermoregulate, they often experience energetic tradeoffs, as warmer temperatures elevate metabolic costs, but also increase their ability to consume more (Magnuson et al. 1979). Some habitats might be avoided due to suboptimal temperatures, either too cold to allow sufficient digestion, or too warm and metabolically demanding (Armstrong et al. 2013; Manderson et al. 2014). Conversely, prey density can influence habitat preference. If prey is scarce, individuals may seek cooler environments to reduce metabolic costs and conserve energy (Brandt 1993). Thus, the services that habitats provide depend on a multitude of interacting abiotic and biotic factors, including the species’ physiology. It is important to investigate how these environmental tradeoffs affect species performance in order to identify critical habitat for the recovery of imperiled species.
Of particular relevance to the North American Pacific Coast is the green sturgeon (Acipenser medirostris), a long-lived, anadromous fish whose populations experience a variety of negative anthropogenic impacts (Adams et al. 2007). The Northern distinct population segment (DPS), which spawns in two river systems (Klamath, California (CA), and Rogue, Oregon (OR)), was listed as a species of concern by NOAA Fisheries. The Southern DPS, spawning only in the highly-modified Sacramento River, CA, is listed under the Endangered Species Act as threatened (Adams et al. 2007). After reaching sexual maturity at 13–20 years of age, individuals spawn in natal streams every 2–5 years thereafter (Moyle 2002; Van Eenennaam et al. 2006; Erickson and Webb 2007).
Among sturgeon species, green sturgeon are particularly marine-oriented and wide-ranging. Despite the small number of natal streams, sturgeon from both distinct population segments range along the Pacific Coast between Mexico and the Bering Sea of Alaska (Moyle 2002). Results from acoustic tagging show individuals move northward in winter, and appear to aggregate north of Vancouver Island, British Columbia (Lindley et al. 2008). During summer and early fall (primarily from May through September), subadult and adult green sturgeon enter estuaries and streams along the U.S. West Coast, where they have been documented through direct capture and via detection of acoustically-tagged individuals (Kelly et al. 2007; Moser and Lindley 2007). Genetic analyses indicate the majority of green sturgeon observed in Willapa Bay, a Washington State estuary (WA), are from the threatened Southern DPS (Schreier et al. 2016).
While in the estuary, green sturgeon consume burrowing shrimp (Neotrypaea californiensis), which dominate stomach contents (Dumbauld et al. 2008). N. californiensis is a habitat engineer whose presence at high densities defines an estuarine habitat type (“shrimp beds”, Ferraro and Cole 2011). High densities of burrowing shrimp coincide with areas where green sturgeon have been observed, along with likely sturgeon feeding pits (Moser et al. in press). By burrowing, N. californiensis destabilizes the sediment, causing burial and suffocation of sessile bivalves. For the past several decades, commercial shellfish growers have applied pesticides to their intertidal aquaculture beds to reduce populations of burrowing shrimp (Feldman et al. 2000). Thus, there are conflicting uses of Willapa Bay’s estuarine tidal flats. Abundant shrimp are important prey for a threatened species, yet a paucity of shrimp is preferred for productive shellfish aquaculture. These opposing drivers influence burrowing shrimp management in Willapa Bay and motivate an exploration of green sturgeon consumption requirements, measured in numbers and biomass of shrimp.
Numerous species of sturgeon display patterns of seasonal growth and fasting, where feeding during the growing season must compensate for metabolic costs during the rest of the year (Sulak and Randall 2002). The consistent recurrence of green sturgeon in estuaries suggests that estuarine habitats provide an important opportunity for growth. For such a large, migratory species like green sturgeon, bays and estuaries may provide the majority of energy and growth needed to sustain individuals throughout the year. During the period that sturgeon occupy Willapa Bay (May through September), here called estuarine residence, mean estuary water temperatures exceed 15 °C and are more than 2 °C warmer than coastal ocean temperatures (Moser and Lindley 2007). Despite the apparent importance of estuaries for green sturgeon, the growth potential of, and the feeding rate and consumption required by green sturgeon occupying estuaries have not been evaluated.
In this study, we use a bioenergetics modeling approach to evaluate the feeding of green sturgeon during estuarine residence and to investigate how warmer estuarine temperatures relative to the coastal ocean influence opportunity for growth. We adapted parameters from an existing white sturgeon (A. transmontanus) bioenergetics model to estimate the feeding and consumption rates required by green sturgeon to achieve their observed annual growth over a range of temperatures, spawning frequencies, and durations of estuarine residence. With these model simulations, we identified environmental conditions and life history strategies that substantially affect the energetic requirements of green sturgeon. Model results help frame the potential consequences of warming or changes in prey availability on the benefit of estuaries to this imperiled species.
Methods
Parameterization of the bioenergetics model
We estimated the consumption rates of green sturgeon under different temperature, spawning, and duration of residence scenarios in Willapa Bay using the Wisconsin Bioenergetics Model parameterized for white sturgeon (Hanson et al. 1997; Bevelhimer 2002) and fit to the observed annual growth of green sturgeon. The model was implemented in R (Development Core Team, 2013). When several parameterizations were plausible, we chose those that would generate larger consumption estimates (“liberal”), given concerns about estuarine habitat transformation, and/or explored the sensitivity of the model output to a range of parameterizations. The central bioenergetics formula is an energy balance equation that relates consumption to growth, metabolic demand, and energy lost in waste, based on body mass- and temperature-dependent functions:
Annual growth of green sturgeon was based on observations of length-at-age for fish collected in Washington and Oregon and aged by pectoral fin-spine sections (N = 307; Farr et al. 2002). The annual growth increments for different age-classes of green sturgeon were computed using a von Bertalanffy growth function fit to the length-at-age data (Farr et al. 2002):
where FL is fork length in cm and t represents age in years. Length-at-age of green sturgeon collected in the Klamath River, CA (USFWS 1983) was comparable to fish collected in WA and OR (Fig. 1), but a similar von Bertalanffy growth function indicated that young fish from the Klamath are slightly shorter and old fish are slightly longer than fish from Farr et al. (2002). Because the bioenergetics model is mass-specific, we converted FL’s to mass (W) using a length-weight regression developed by Van Eenennaam et al. (2006):
von Bertalanffy growth functions describing the length-at-age for green sturgeon captured in Washington (WA), Oregon (OR), and California (CA). For WA and OR (solid line), fish were sampled from Puget Sound, the Columbia River, Yaquina Bay, Winchester Bay, Coos Bay, and the Rogue River 1949–2002 (N = 307, Farr et al. 2002). For CA (dashed line), fish were sampled from the Klamath River 1979–1982 (N = 173, USFWS, 1983), and converted from total length (TL) to fork length (FL): TL = −4.6131 + 1.1374 (FL) (Nakamoto et al. 1995)
To generate liberal consumption estimates, we chose the length-weight relationship derived from female sturgeon, which yields individuals that are slightly heavier than males at comparable lengths. The somatic energy density for green sturgeon was set to 4393.2 J g−1 for all age classes, converted from 1050 cal g−1 wet weight for mixed sturgeon species (USDA 2001). Because the USDA reports nutrition content based on edible portions only, our estimate of somatic energy density is likely higher than for whole-body sturgeon and should generate liberal consumption estimates. It is worth noting that another length-weight formula estimates much heavier fish per length, by as much as a factor of two for fish sampled in the Klamath River (Nakamoto et al. 1995), relative to Eq. (3). However, the Van Eenennaam et al. (2006) relationship was similar to body mass and length measurements from other unpublished works (O. Langness, Washington Department of Fish and Wildlife, pers. comm.).
The energy required for respiration (R) was computed based on the body mass of green sturgeon, temperature (T), activity level (ACT), and costs associated with digesting food, termed specific dynamic action (SDA):
where RA, RB, and RQ are parameters describing the allometric and temperature dependent components of respiration (Table 1). These parameters were estimated from several laboratory studies that used different body sizes of white sturgeon held at different temperatures (Bevelhimer 2002). We modified Eq. (4) from that of Bevelhimer (2002) by multiplying RA by 4.184 to convert from calories to joules and subtracting 1 from RB to make the equation mass specific and align with other models. Specific dynamic action (SDA), egestion (F), and excretion (U) in Eq. (1) were assumed to increase as constant proportions of consumption (Table 1). We used an activity rate multiplier ACT of 2, which assumes that green sturgeon activity doubles their metabolic cost above their basal metabolic rate. This level of activity was first suggested by Winberg (1956) as a lifelong average for fish species and is comparable to other species of sturgeon (ACT = 1.5, Niklitschek and Secor 2005). Because no significant differences in respiration rate were found in juvenile green sturgeon held at salinities ranging from <3 to 33 ppt (Allen and Cech 2007) we felt respiration rates estimated from Eq. (4) were suitable for green sturgeon occupying both the reduced salinities of Willapa Bay and the coastal ocean (20–32 PSU; Moser and Lindley 2007).
Daily consumption rates for green sturgeon were estimated as a proportion (P) of the maximum theoretical daily consumption rate (Cmax) for white sturgeon of a given size and at a given temperature such that predicted growth from the model would match observed growth after accounting for energy lost to metabolism and waste (Bevelhimer 2002):
where CA and CB are parameters describing the dependence of maximum consumption rate on fish mass and f(T) represents a function describing dependence on temperature. The equation for f(T) follows that of Kitchell et al. (1977) and was derived with an optimal feeding temperature of 20 °C and a maximum of 28 °C (see Bevelhimer 2002). Relative to Bevelhimer (2002), Eq. (6) includes subtracting 1 from CB to make the equation mass specific. Although parameters for Eq. (6) were developed for white sturgeon, food conversion efficiency (weight gain per amount of food consumed) as a function of temperature is similar between green and white sturgeon, supporting the use of a white sturgeon model for this analysis (Mayfield and Cech 2004).
Because Mayfield and Cech (2004) found that the respiration rates of juvenile green sturgeon were higher than those of other sturgeon species, we investigated the sensitivity of parameters in the bioenergetics model by performing an individual parameter perturbation analysis (Bartell et al. 1986; Table 1). Simulations were conducted for an age-20 sturgeon. We individually and sequentially decreased and increased the value of each parameter by 10%. We calculated the percent change in the estimated consumption rate after adjusting each parameter. Results from this analysis indicated that consumption rates are particularly sensitive to small changes in the respiration parameters RB and RQ. A 10% change in RB and RQ resulted in changes of consumption rate by up to 24% and 12%, respectively.
Diet composition
Green sturgeon are benthic feeders (Moyle 2002; Dumbauld et al. 2008), but their diets are not well documented. Based on gut contents of sturgeon caught as bycatch in the summer 2003 Willapa Bay salmon fishery (N = 9), burrowing shrimp Neotrypaea californiensis were present in more than half of the fish and represented 89% of identifiable gut contents by mass (Dumbauld et al. 2008). To estimate the maximum likely consumption rate of burrowing shrimp for individual green sturgeon, all model simulations assumed a diet composition of 100% burrowing shrimp.
We estimated the energy density of burrowing shrimp with bomb calorimetry on whole body samples of N. californiensis, collected in summer 2015 at 10 locations throughout Willapa Bay. No significant trends in the energy density of shrimp were detected over time or among sizes of shrimp across the estuary. We assumed no spatial effects, as each site was sampled on a different date. Additionally, no sex-based differences in energy density were found (Student’s T test, N = 37, P = 0.893). Qualitative assessment of studentized-deleted residuals showed no departures from normality. Therefore, a mean energy density of 4842 J g−1 wet weight was used for model simulations; variation around this energy density was small (SD = 1.14 kJ g−1) and not incorporated into simulations. On average, burrowing shrimp sampled from the bay weighed 5.68 g (SD = 3.12 g, N = 1193; Washington Department of Natural Resources, unpublished data) and contained 27,511 J.
Thermal experience
Estuarine temperature was measured in surface water at Port of Peninsula docks in Willapa Bay (46.501°N, 124.030°W) at 15–60 min intervals in 2004–2008 (Washington Department of Fish and Wildlife, unpubl. Data), and these values were used to generate daily average water temperatures for 1 May through September (day of year 121–273). These data were described by fitting a second-order polynomial regression to provide a daily input temperature for bioenergetics simulations (Fig. 2). Ocean water temperature was measured 0.6 m below water line at the Columbia River Lightship (46.82°N, 124.14°W, http://www.nodc.noaa.gov/dsdt/cwtg/all_meanT.html; retrieved May 6, 2016) and available as bi-weekly or monthly averages ranging from 8.3 °C in winter to 14.4 °C in summer (Fig. 2).
Daily water temperatures measured at Port of Peninsula in Willapa Bay from May through September 2004–2008 (days 121–273; gray points, fit by second order polynomial regression), and semimonthly means measured in the coastal ocean from the Columbia River Lightship (dashed line). Bracket indicates the average entry (Day of year = 198) and duration (34 days) of acoustically-tagged individuals detected in Willapa Bay 2005–2008
Bioenergetics simulations
We simulated the per-capita consumption and growth for ages 10–25 green sturgeon (FL 110–160 cm) in annual increments (simulation day 1 = January 1st) with the bioenergetics model. These size and age groups correspond to those commonly observed in Willapa Bay (Moser and Lindley 2007). Simulations were designed to capture the thermal environment of Willapa Bay and variation in the life history of green sturgeon, represented here as variation in thermal experience, reproductive investment, and estuarine residence (see below). Model results allowed us to assess how changes in thermal experience, spawning, and time spent feeding in Willapa Bay influence the consumption rate required for green sturgeon to achieve their observed growth. Because seasonal feeding in estuaries may explain how sturgeon meet growth requirements (Sulak and Randall 2002), and in order to estimate upper bounds of burrowing shrimp consumption, we assumed feeding on burrowing shrimp during estuarine residence supports 100% of the annual growth for different age-classes of green sturgeon. During other parts of the year in the colder coastal ocean, we assumed feeding on alternative prey is sufficient to support metabolic demand and maintain body weight.
Variation in thermal experience
We simulated four different temperature scenarios from May through September to capture variation in the thermal experience of green sturgeon moving between the coastal ocean and Willapa Bay: 1) observed water temperatures in the ocean, 2) one standard deviation below daily mean water temperatures measured in Willapa Bay (2 °C lower than average), 3) daily mean water temperatures measured in Willapa Bay, and 4) one standard deviation above daily mean water temperatures measured in Willapa Bay (2 °C higher than average) (Fig. 2). The four temperature regimes thus provided different estimates of the consumption rate required to meet metabolic demands for five months of estuarine residence and to achieve the entire annual growth increment. Because it is possible that feeding conditions for green sturgeon in the ocean may not be sufficient to maintain body weight, we explored the case in which green sturgeon fast (and lose weight) outside of estuaries. Therefore, feeding in Willapa Bay must support this deficit and total annual growth. We simulated this alternative by applying temperature scenario 1 (above) for September to May and scenario 3 for May through September.
Variation in reproductive investment
Additional simulations under the average temperature scenario (3 above) were conducted for an age-20 male and female green sturgeon to assess how variation in spawning frequency (every 2–5 years; Moyle 2002) influences energetic requirements while feeding in Willapa Bay. Because spawning by an individual does not occur every year, we adjusted the somatic energy density of green sturgeon for our annual model simulations, based on the energy density of gonads and total gonadal weight at the time of spawning, to reflect the longer-term maturation schedule. Mature male and female green sturgeon gonads represent 5.2% and 12.6% of their total body mass, respectively (Van Eenennaam et al. 2006). To model the range of spawning frequencies indicated in the literature (Moyle 2002), the sex-specific amount of gonadal tissue at time of spawning was divided by either 2 or 5 and multiplied by gonadal energy density (9204.8 J/g; Bevelhimer 2002) to compute the annual energy required to support gonad growth. The remaining amount of somatic mass was multiplied by the somatic energy density used above (4393.2 J g−1). Together, these computations resulted in composite sturgeon energy densities of 4518.3 J g−1 for male and 4696.33 J g−1 for female sturgeon spawning every 2 years, and 4443.24 J g−1 for male and 4514.45 J g−1 for female sturgeon spawning every 5 years.
Variation in the duration of estuarine residence
Thus far, we have assumed individual green sturgeon occupy Willapa Bay for the entire May to September period (153 total days) to provide liberal expectations for consumption in the estuary. Next, we modeled consumption rates for an individual age-20 sturgeon of known residence time, based on estimates from an acoustic telemetry study. Adult and subadult green sturgeon >99 cm FL were captured from San Pablo Bay, CA to Grays Harbor WA in 2003–2005 and each fish was outfitted with a uniquely-coded acoustic transmitter having a 4-year battery life (Lindley et al. 2008). Use of Willapa Bay by tagged green sturgeon was documented by acoustic detections recorded during 2005–2008. During this time, 92 acoustically-tagged individuals were detected by at least one of 12 receivers deployed on navigation aids throughout Willapa Bay. We dropped from consideration any individuals whose detections only occurred within 24 h, as these fish were not considered to have fully utilized the estuary. Additionally, we censored individuals whose estuarine residence was interrupted by excursions outside of Willapa Bay (indicated by detections in the Columbia River and Grays Harbor estuaries), leaving a sample size of 89 fish when summed across years.
On average, tagged green sturgeon entered on July 17 and spent 34 days in the estuary (SD = 41 days). We simulated the consumption and growth of an age-20 sturgeon entering on July 17 and residing for 34 or 75 days (mean + 1 SD). We also examined the outcomes of shorter residence durations of 8 and 15 days. Within the five-month simulation period, days before and after estuarine residence were simulated at corresponding coastal ocean temperatures, when feeding was assumed to offset metabolic demand. Like before, these simulations assumed feeding in the estuary was sufficient to support the entire observed annual growth increment for green sturgeon, despite the now shorter residence times as informed by acoustic telemetry. Total consumption of burrowing shrimp estimated for the different residence times (including a residence of 153 days for an entry date of May 1st) was partitioned into the fractions needed to satisfy metabolic demand (in the ocean and estuary) and the annual growth increment. To explore the consequences of longer estuarine residence on growth, daily ratios of weight gain to consumption for each scenario of estuarine residence were averaged to compare growth efficiencies between different durations of residence in Willapa Bay.
Results
Effect of thermal experience on consumption
The estimated consumption of burrowing shrimp required to achieve the annual growth increment for green sturgeon feeding in Willapa Bay for 153 days (May–September) ranged from 13.0 to 18.3 kg for a 10-year old individual and 22.8 to 33.1 kg for a 25-year old individual from the coolest (ocean) to warmest thermal regime (2 °C above the average for Willapa Bay; Fig. 3). These simulations assumed that green sturgeon consumption in the ocean from September to May was sufficient to maintain body weight when not feeding in Willapa Bay. Conversely, under the average thermal regime for Willapa Bay and the assumption that green sturgeon fast, and therefore lose weight while in the ocean, the consumption that was needed to recover from this deficit and achieve the annual growth increment for each age-class was much higher and ranged from 28.5 to 53.0 kg burrowing shrimp for individuals age-10 to 25 years (Fig. 3). For the remaining simulations, we used the average thermal regime of Willapa Bay and assumed feeding by green sturgeon while in the ocean was sufficient to maintain body weight.
Estimated consumption by green sturgeon feeding in Willapa Bay for 153 days from May through September under different thermal regimes (described in Methods). Consumption rates for ‘Feeding in Willapa, fasting in ocean’ assume that green sturgeon fast and lose weight from October to May in the coastal ocean and therefore must compensate by feeding in Willapa Bay at increased rates. Warm, Average, and Cool Willapa refer to simulations at 1 standard deviation above, at, and below average temperatures of Willapa Bay, respectively. In these simulations, feeding outside of estuaries from October to May is expected to meet metabolic costs. Ocean refers to consumption (in units of burrowing shrimp) at coastal ocean temperatures, measured at Columbia River Lightship
Effect of reproductive investment on consumption rate
Incorporating gonadal growth over 2 or 5 years into the 153-day residence time simulations in Willapa Bay had a negligible effect on the consumption estimated for a reproductive age-20 individual when compared to simulations that did not include reproductive investment. For an age-20 male green sturgeon, consumption only increased by 0.15% (25.92–25.88 kg) for a spawning frequency of 2 years, and by 0.06% (25.90–25.88 kg) for a spawning frequency of 5 years. Similarly, for an age-20 female, consumption only increased by 0.37% (25.98–25.88 kg) and 0.15% (25.92–25.88 kg) for spawning frequencies of 2 and 5 years, respectively.
Effect of estuarine residence time and entry date on feeding and consumption rate
The average temperature of Willapa Bay varied over the growing season, rising from 12.5 °C in May to a peak of 18.4 °C in August, and then cooling to 16.0 °C toward the end of September (Fig. 2). Consequently, the thermal regime experienced by green sturgeon varied depending on the entry date and duration of residence modeled. The average daily temperature experienced by a green sturgeon remaining in the ocean from May through September was 13.4 °C and increased with longer durations of estuarine residence. Mean thermal experience was 16.9 °C for a fish entering the bay on May 1st and residing for 153 days, thereby experiencing the full 5 months of temperatures in the estuary. Conversely, simulations of fish entering the bay on July 17th (mean entry date estimated from telemetry) and residing for decreasing durations of 75, 34, 15, and 8 days experienced slightly higher average temperatures within Willapa Bay (17.8 °C for 75 d and 18.3 °C for shorter durations). This entry date, combined with shorter residence, corresponded to when temperatures in the bay were at their peak (Fig. 2).
Because each simulation was constrained to the same observed growth increment, the average daily feeding rate, or proportion (P) of maximum consumption rate (Cmax), declined as duration increased, since growth accrued over longer periods (Fig. 4a). A green sturgeon spending 8 days in the estuary required 61 shrimp d−1 (96% Cmax) to achieve the observed annual growth increment, whereas sturgeon required 38 shrimp d−1 (60% Cmax) for 34 days (average duration) and 30 shrimp d−1 (50% Cmax) for 153 days (Fig. 4a). Feeding rate exceeded 100% Cmax for durations of less than 8 days. Therefore, these simulations reflect a continuum of alternative feeding strategies ranging from (1) entering the estuary in spring when temperatures are warming and residing and growing over a longer period while feeding at a reduced rate to (2) entering the estuary later when temperatures are near their summer peak, feeding at a higher rate, and growing more quickly over a shorter period. The consumption estimated for different durations of estuarine residence was partitioned into the fractions required to satisfy metabolic demand in the ocean versus the estuary and the annual growth increment (Fig. 4b). Overall, increasing the duration of residence from 8 days to 153 days in Willapa Bay led to a 24% increase in estimated consumption for an age-20 green sturgeon from May through September. In this study, we refer to consumption as the total biomass of food consumed over a specified time interval.
Results from bioenergetics simulations for a 20-year old green sturgeon feeding for various durations in Willapa Bay from May through September (153 days). Estuary entry dates were July 17 (day of year = 198) for residence durations of 8, 15, 34, and 75 days and May 1 (day of year = 121) for 153 days. a Proportion of maximum consumption (analogous to feeding rate) to meet metabolic demand and the annual growth increment for complete oceanic residence (0 days duration in Willapa Bay) and for varying durations of residence in Willapa Bay (8, 15, 34, 75, 153 days). b Estimated consumption partitioned into fractions needed to satisfy metabolic demand (in the ocean versus estuary) and the annual growth increment for corresponding durations of residence in Willapa Bay
Growth associated with alternative feeding strategies
Growth efficiency (ratio of weight gain to consumption) decreased as duration of residence in Willapa Bay increased. Growth efficiency was highest for durations of 8 days (39%). However, an age-20 green sturgeon needed to feed near its maximum theoretical capacity (near 100% Cmax) each day to achieve annual growth over this short period. Sustaining such a high feeding rate is unlikely given that green sturgeon are often observed in the estuary for longer periods. Growth efficiency for the average observed residence time of 34 days was 15%, higher than the efficiencies calculated for 75 days (8%) and 153 days (4%) of residence.
Discussion
By adapting a white sturgeon bioenergetics model, we estimated the consumption demand, in units of burrowing shrimp, for individual green sturgeon to satisfy age-specific annual growth increments under different thermal regimes, reproductive strategies, and durations of estuarine residence. Assuming prey is sufficiently available, model simulations indicated that green sturgeon could satisfy their entire observed annual growth increment by feeding in Willapa Bay for at least 8 days during the spring and summer. We estimated the maximum annual consumption requirement of an age-20 green sturgeon residing in Willapa Bay to be 8299 burrowing shrimp, assuming it must make up for weight lost while fasting outside of the bay. This requirement increased for larger, older sturgeon, but at a decreasing rate because mass-specific feeding capacity decreases faster than mass-specific metabolic demand, which reduces growth potential (Beauchamp 2009). Consumption demand was lower (4556 burrowing shrimp, 20-year old fish) if green sturgeon obtained sufficient resources outside estuaries to meet their metabolic costs and maintain weight. We found that variation in the thermal environment, whether modeled directly through assumptions about estuarine temperature, or indirectly through manipulating entry date and duration of residence, had large effects on modeled consumption and growth efficiency. Conversely, we found that reproductive investment by males and females across a range of likely spawning frequencies did not have a large effect on per-capita consumption rate. Below, we address the assumptions and implications of these factors in turn. Finally, we apply our results to estimate the number of green sturgeon that can be supported by burrowing shrimp populations in Willapa Bay.
As duration of estuarine residence decreased, growth efficiency increased. Among scenarios of feeding in Willapa Bay, a duration of 8 days at peak Willapa Bay temperatures was the best strategy in terms of growth efficiency. Increased digestive capacity resulting from warm temperatures made it possible to meet the annual growth increment over only a few days despite the elevated metabolic costs. According to the bioenergetics model however, this feeding strategy pushed against physiological digestive constraints since green sturgeon needed to feed very close to Cmax. Thus, this feeding strategy is highly contingent on sufficient prey availability. Conversely, results suggested that a duration of 153 days was inefficient due to prolonged exposure to warm temperatures and relatively high metabolic rates. However individuals in these simulations could still meet their annual growth increment by feeding at a lower rate, which might be advantageous if prey is scarce.
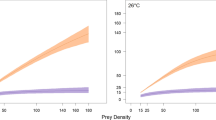
Over the range of temperatures currently experienced by green sturgeon in Willapa Bay and the coastal ocean, warmer temperatures accelerate both metabolism and maximum consumption, through temperature-dependent digestive capacity. However, a sturgeon’s ability to achieve maximum consumption also hinges on prey availability. Accordingly, in simulations that span a range of consumption rates, higher feeding rates required higher temperatures to maximize growth potential. Green sturgeon feeding at 75–100% Cmax maximized growth potential at 18 °C, whereas growth potential at 50% Cmax peaked at 16 °C (Fig. 5). These simulations corroborated the continuum of alternative feeding strategies mentioned above. If prey are less abundant, entering Willapa Bay early when waters are relatively cooler provides greater growth potential because metabolic costs are lower. Conversely, if prey are sufficiently abundant, entering Willapa Bay when temperatures are near their peak provides greater growth potential despite higher metabolic costs because of the corresponding increase in digestive capacity. However, small increases in temperature beyond the peak values observed can limit growth potential considerably, particularly at feeding rates >75% Cmax (Fig. 5).
Simulations of estimated mass-specific growth rate of an age-20 green sturgeon, feeding at fixed proportions of maximum consumption (Cmax) across a range of temperatures. Maximum consumption increases with temperature. Light and dark grey shading indicate the full range and mean range of temperatures, respectively, corresponding to the average observed green sturgeon entry date and duration of residence in Willapa Bay informed by telemetry (entry date = July 17th, duration = 34 days)
The average estuarine residence of 34 days (observed from acoustically-tagged fish) appears optimal in terms of balancing metabolic costs and digestive capacity, given the ambient availability of prey. Simulations of feeding in Willapa Bay for 34 days resulted in a comparable feeding rate (proportion of maximum consumption) to longer durations of residence but had a growth efficiency that was 3.6 times higher than feeding for 153 days in the estuary. In addition, average observed dates of estuarine residence show that individuals enter Willapa Bay during its thermal maximum, when metabolic costs–but also digestive capacity–are highest (dark grey shading, Fig. 5). This suggests that individuals modify their thermal environment and that most fish adopt a strategy of shorter estuarine residence with more intense feeding. These results also suggest that individuals may not be able to acquire their annual growth requirement over periods of <34 days, whether due to the contemporary availability of prey or other feeding constraints. Therefore, if temperature increases and prey availability remains the same, scope for growth may diminish. Furthermore, if temperature increases and prey abundance declines, this could have severe consequences for green sturgeon growth.
Of all factors examined, the estimated per-capita consumption rates for green sturgeon were most sensitive to estuarine temperature. Simulations at the warmest Willapa Bay temperatures explored (avg. +1 SD) required that green sturgeon consume at least 40% more burrowing shrimp than simulations at relatively cool coastal ocean temperatures. This increased intake reflected the additional energy required to satisfy the higher metabolic cost of warmer temperatures. Parameters related to respiration rate also influenced results, as revealed through our sensitivity analyses. The allometric slope (RB) of the respiration function exhibited particularly high sensitivity in the model, whereby a ± 10% change in the parameter value resulted in up to a ± 24% change in the estimated consumption rate. It is possible that green sturgeon have higher respiration rates than white sturgeon (the species used to parameterize the model), possibly due to greater red muscle tissue mass (Mayfield and Cech 2004). Therefore, until sufficient laboratory experiments can be conducted to parameterize and corroborate a bioenergetics model specific to green sturgeon, management decisions based on the consumption rates estimated in the present study should remain conservative by incorporating this additional uncertainty.
Estimated consumption was relatively insensitive to assumptions about spawning frequency. Because individuals had between two and five years to assimilate the relatively small proportion of body mass as gonadal growth (12.6% in females) within our modeling framework, the influence of reproductive investment on total energy intake was negligible, increasing by a maximum of 0.37% for females spawning every two years. This result highlights one potential advantage of being a large-bodied species that spawns infrequently; interannual variation in prey availability may not adversely affect reproductive output. Even though green sturgeon appear to spawn every 2 to 5 years, it is possible that most gonad development occurs over a shorter period within the spawning year. If so, the effect of reproductive investment on annual energy intake would be greater than indicated by our simulations. Our simulations of reproductive investment do not include energy required to synthesize gonads or undergo spawning migrations. Therefore, in contrast to other assumptions we are making, more detailed physiological data associated with reproduction could increase consumption requirements. Consumption estimates will scale, such that if gonad synthesis and spawning migrations double the total energy relative to that included in gonadal tissue, the fraction of consumption allocated to reproduction will also double, but would still remain small. Information regarding the reproductive phenology of green sturgeon is limited. Future research should address this uncertainty with empirical measurements of gonadal mass or hormonal markers from maturing individuals with appropriate temporal resolution and in appropriate habitats (McKinley et al. 1998).
Determining whether sufficient burrowing shrimp are available for current and future green sturgeon populations is critical for resource management. The bioenergetics approach taken here is an essential component of estimating carrying capacity because it provides a mechanistic underpinning for evaluating burrowing shrimp consumption by individual green sturgeon. The maximum intake rests on assumptions that green sturgeon are specialists on burrowing shrimp (consuming 8299 shrimp y−1 for an age-20 fish), reside 153 days y−1 in Willapa Bay, and fast outside of the estuary. Two methods involving bay-wide shrimp biomass estimates, scaled by a production per biomass ratio of 0.3 (Banse and Mosher 1980), have been used to estimate the annual production of burrowing shrimp in Willapa Bay (Supplemental material). Standing stock of 2.33–7.56 · 106 kg would produce enough burrowing shrimp to support 14,800–48,000 age-20 green sturgeon. Our estimate of estuarine capacity likely meets or exceeds the current population size of the southern DPS of green sturgeon using Willapa Bay each year (Moser et al. 2016). This analysis provides a preliminary reference point of broad confidence to guide near-term management and should be used with caution. The calculations assume green sturgeon are the only significant predator of burrowing shrimp (and feed exclusively on burrowing shrimp in the bay) and that shrimp consumed in the subtidal areas represent an insignificant portion of the entire shrimp biomass that sturgeon rely upon.
Fishes have adapted to move among different habitats in order to increase their rates of growth, survival, and reproduction. These movements can be complex due to tradeoffs between physiology and environmental abiotic and biotic factors. For imperiled species, it is important to examine the beneficial conditions of preferred habitats to inform management and recovery planning. In this study, we applied a bioenergetics model to investigate how variation in green sturgeon life history affects consumption rates and growth potential during seasonal occupation of Willapa Bay. Our results show that date of entry, duration of residence, and thermal experience have substantial effects on the consumption rate and growth efficiency of green sturgeon. Differences in the consumption rate and growth efficiency associated with each feeding strategy examined were driven by the interplay among thermal experience, metabolic demand, and digestive capacity. Simulation results along with observed entry dates and residence times from acoustically-tagged individuals suggest that green sturgeon take advantage of increased digestive capacity by entering the bay when temperatures are near their peak, despite the greater metabolic cost of occupying those temperatures. However, the growth potential associated with this strategy was highly sensitive to prey availability/feeding rate and small increases in temperature. Thus environmental or anthropogenic driven changes in the thermal regime of coastal estuaries, reductions in prey availability, or both could present new challenges to recovering green sturgeon populations.
References
Adams PB, Grimes C, Hightower JE, Lindley ST, Moser ML, Parsley MJ (2007) Population status of North American green sturgeon, Acipenser medirostris. Environ Biol Fish 79:339–356
Allen PJ, Cech JJ Jr (2007) Age/size effects on juvenile green sturgeon, Acipenser medirostris, oxygen consumption, growth, and osmoregulation in saline environments. Environ Biol Fish 79:211–229
Armstrong JB, Schindler DE, Ruff CP, Brooks GT, Bentley KE, Torgersen CE (2013) Diel horizontal migration in streams: juvenile fish exploit spatial heterogeneity in thermal and trophic resources. Ecology 94:2066–2075
Banse K, Mosher S (1980) Adult body mass and annual production/biomass relationships of field populations. Ecol Monogr 50:355–379
Bartell SM, Breck JE, Gardner RH, Brenkert AL (1986) Individual parameter perturbation and error analysis of fish bioenergetics models. Can J Fish Aquat Sci 43:160–168
Beauchamp DA (2009) Bioenergetic ontogeny: linking climate and mass-specific feeding to life-cycle growth and survival of salmon. Am Fish Soc Symp 70:1–19
Bevelhimer MS (2002) A bioenergetics model for white sturgeon Acipenser transmontanus: assessing differences in growth and reproduction among Snake River reaches. J Appl Ichthyol 18:550–556
Brandt SB (1993) The effect of thermal fronts on fish growth: a bioenergetics evaluation of food and temperature. Estuaries 16:142–159
Clark CW, Levy DA (1988) Diel vertical migrations by juvenile sockeye salmon and the antipredation window. Am Nat 131:271–290
Dumbauld BR, Holden DL, Langness OP (2008) Do sturgeon limit burrowing shrimp populations in Pacific northwest estuaries? Environ Biol Fish 83:283–296
Erickson DL, Webb MAH (2007) Spawning periodicity, spawning migration, and size at maturity of green sturgeon, Acipenser medirostris, in the Rogue River, Oregon. Environ Biol Fish 79:255–268
Farr RA, Hughes ML, Rien TA (2002) Green sturgeon population characteristics in Oregon. Annual Progress Report Sport Fish Restoration Project F-178-R
Feldman KL, Armstrong DA, Dumbauld BR, DeWitt TH, Doty DC (2000) Oysters, crabs, and burrowing shrimp: review of an environmental conflict over aquatic resources and pesticide use in Washington state’s (USA) coastal estuaries. Estuaries 23:141–176
Ferraro SP, Cole FA (2011) Ecological periodic tables for benthic macrofaunal usage of estuarine habitats in the US Pacific northwest. Estuar Coast Shelf Sci 94:36–47
Glover DC, DeVries DR, Wright RA (2013) Growth of largemouth bass in a dynamic estuarine environment: evaluation of the relative effects of salinity, diet, and temperature. Can J Fish Aquat Sci 70:485–501
Hanson PC, Johnson TB, Schindler DE, Kitchell JF (1997) Fish bioenergetics 3.0. University of Wisconsin Sea Grant Institute, technical report WISCU-T-97-001, Madison
Kelly JT, Klimley AP, Crocker CE (2007) Movements of green sturgeon, Acipenser medirostris, in the San Francisco Bay estuary, California. Environ Biol Fish 79:281–295
Kitchell JF, Stewart DJ, Weininger D (1977) Applications of a bioenergetics model to yellow perch (Perca flavescens) and walleye (Stizostedion vitreum vitreum). J Fish Res Board Can 34:192201935
Lindley ST, Moser ML, Erickson DL, Belchik M, Welch DW, Rechisky EL, Kelly JT, Heublein J, Klimley AP (2008) Marine migration of north American green sturgeon. Trans Am Fish Soc 137:182–194
Magnuson JJ, Crowder LB, Medvick PA (1979) Temperature as an ecological resource. Am Zool 19:331–343
Manderson JP, Stehlik LL, Pessutti J, Rosendale J, Phelan B (2014) Residence time and habitat duration for predators in a small mid-Atlantic estuary. Fishery Bulletin 112:144–159
Mayfield RB, Cech JJ Jr (2004) Temperature effects on green sturgeon bioenergetics. Trans Am Fish Soc 133:961–970
McKinley S, Van Der Kraak G, Power G (1998) Seasonal migrations and reproductive patterns in the lake sturgeon, Acipenser fulvescens, in the vicinity of hydroelectric stations in northern Ontario. Environ Biol Fish 71:245–256
Moser ML, Lindley ST (2007) Use of Washington estuaries by subadult and adult green sturgeon. Environ Biol Fish 79:243–253
Moser ML, Israel JA, Neuman M, Lindley ST, Erickson DL, McCovey BW, Klimley AP (2016) Biology and life history of green sturgeon (Acipenser medirostris Ayres, 1854): state of the science. J Appl Ichthyol 32:67–86
Moser ML, Patten K, Corbett SC, Feist BE, Lindley ST (in press) Abundance and distribution of sturgeon feeding pits in a Washington estuary. Environ Biol Fish
Moyle PB (2002) Inland fishes of California. University of California Press, Berkeley
Nakamoto RJ, Kisanuki TT, Goldsmith GH (1995) Age and growth of Klamath River green sturgeon (Acipenser medirostris). U.S. Fish and Wildlife Service report 93-FP-13, Yreka
Niklitschek EJ, Secor DH (2005) Modeling spatial and temporal variation of suitable nursery habitats for Atlantic sturgeon in the Chesapeake Bay. Estuar Coast Shelf Sci 64:135–148
R Development Core Team (2013) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna http://www.R-project.org. Accessed Mar 2015
Schreier A, Langness OP, Israel JA, Van Dyke E (2016) Further investigation of green sturgeon (Acipenser medirostris) distinct population segment composition in non-natal estuaries and preliminary evidence of Columbia River spawning. Environ Biol Fish 99:1021–1032
Sulak KJ, Randall M (2002) Understanding sturgeon life history: enigmas, myths, and insights from scientific studies. J Appl Ichthyol 18:519–528
United States Fish and Wildlife Service (USFWS) (1983) Klamath River fisheries investigation, annual report 1982, Arcata
USDA (U.S. Department of Agriculture, Agricultural Research Service) (2001) USDA nutrient database for standard reference, release 14. Nutrient Data Laboratory Home Page, http://www.nal.usda.gov/fnic/foodcomp
Van Eenennaam JP, Linares J, Doroshov SI, Hillemeier DC, Wilson TE, Nova AA (2006) Reproductive conditions of the Klamath River green sturgeon. Trans Am Fish Soc 135:151–163
Winberg GG (1956) Rate of metabolism and food requirement of fishes. Belorussian University, Minsk
Acknowledgements
We thank Mark Bevelhimer at the Oak Ridge National Laboratory Environmental Sciences Division for assistance with implementing his white sturgeon bioenergetics model. Olaf Langness and Steve Lindley provided technical expertise and administrative support. We also thank a number of people who helped sample and process burrowing shrimp including Dr. Alan Trimble, Alazar Dowty, Collin Gross, Aidan Klemmer, Daniel Sund, and Molly Ware. Funding was provided by the Washington State Department of Natural Resources through an interagency agreement with the University of Washington (IAA 16-19). Sturgeon tagging was supported by a Northwest Fisheries Science Center Internal Grant and the National Marine Fisheries Service Candidate Species Program. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(PDF 236 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Borin, J.M., Moser, M.L., Hansen, A.G. et al. Energetic requirements of green sturgeon (Acipenser medirostris) feeding on burrowing shrimp (Neotrypaea californiensis) in estuaries: importance of temperature, reproductive investment, and residence time. Environ Biol Fish 100, 1561–1573 (2017). https://doi.org/10.1007/s10641-017-0665-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10641-017-0665-3