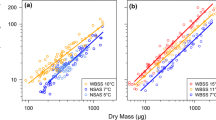
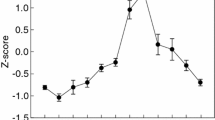
Abstract
Despite their trophodynamic importance in many aquatic ecosystems, few studies have quantified the feeding-growth relationship of clupeid fishes. In laboratory trials, we quantified the relationship between rates of food consumption (C, % fish energy content (Joules d−1)), somatic growth rate (G, % Joules d−1), and swimming speed (S S, body lengths (bl) s−1) for post-larval (30- to 50-mm standard length) European sprat (Sprattus sprattus L.) collected from the southwestern Baltic Sea. Measurements of G and S S were also made on groups before and after an abrupt shift in prey availability. Maintenance (0-growth) and maximum food consumption rates were 5.5 and 42 % somatic energy content d−1, respectively. Mean ± SE gross growth efficiency (K 1 = 100∙G∙C −1) was 26.9 ± 3.0 %. Unfed post-larvae had markedly lower S S compared to continuously-fed fish (0.1 versus 0.5 to 0.7 bl s−1). After 10 days of re-feeding, one group of previously unfed fish was hyperactive (mean S S of 1.2 bl s−1) but no re-fed groups exhibited hyperphagia (based upon prey numbers), increased K 1, or compensatory growth. Increased competition (relatively high S S) was evident during feeding in fish maintained at low to moderate but not at ad libitum prey levels. Our findings provide estimates of prey resources required to fuel in situ growth and help characterize metabolic strategies of European sprat within variable feeding environments.





Similar content being viewed by others
References
Ali M, Nicieza A, Wootton RJ (2003) Compensatory growth in fishes: a response to growth depression. Fish Fish Ser 4:147–190
Alshuth S (1988) Daily growth increments on otoliths of laboratory-reared sprat Sprattus sprattus L. larvae. Meeresforsch 32:23–29
Arndt SKA, Benfey TJ, Cunjak RA (1996) Effect of temporary reductions in feeding on protein synthesis and energy storage of juvenile Atlantic salmon. J Fish Biol 49:247–276
Arrhenius F, Hansson S (1993) Food consumption of larval, young and adult herring and sprat in the Baltic Sea. Mar Ecol Prog Ser 96:125–137
Arrhenius F, Hansson S (1999) Growth of Baltic Sea young-of-the-year herring Clupea harengus is resource limited. Mar Ecol Prog Ser 191:295–299
Bar N, Volkoff H (2012) Adaptation of the physiological, endocrine, and digestive system functions to prolonged food deprivation in fish. In: McCue MD (ed) Comparative physiology of fasting, starvation, and food limitation. doi:10.1007/978-3-642-29056-5_6
Baumann H, Peck MA, Götze E, Temming A (2007) Starving early juvenile sprat Sprattus sprattus (L.) in western Baltic coastal waters: evidence from combined field and laboratory observations in August and September 2003. J Fish Biol 70:853–866
Bejda A, Phelan BA, Studholm AL (1992) The effect of oxygen on the growth of young-of-the-year winter flounder, Pseudopleuronectes americanus. Environ Biol Fish 34:321–327
Björnsson B (1993) Swimming speed and swimming metabolism of Atlantic cod (Gadus morhua) in relation to available food: a laboratory study. Can J Fish Aquat Sci 50:2542–2551
Brachvogel R, Meskendahl L, Herrmann J-P, Temming A (2013) Functional responses of juvenile herring and sprat in relation to different prey types. Mar Biol 160:465–478
Brett JR, Groves TDD (1979) Physiological energetics. In: Hoar WS, Randall DJ, Brett JR (eds) Fish physiology, vol VIII. Academic, New York, pp 279–352
Buckel JA, Letcher BH, Conover DO (1998) Effects of a delayed onset of piscivory on the size of age-0 bluefish. Trans Am Fish Soc 127:576–587
Checkley DM (1984) Relation of growth to ingestion for larvae of Atlantic herring Clupea harengus and other fish. Mar Ecol Prog Ser 18:215–224
Condrey RE (1982) Ingestion-limited growth of aquatic animals: the case for Blackman kinetics. Can J Fish Aquat Sci 39:1585–1595
Cui Y, Hung SSO, Zhu X (1996) Effect of ration and body size on the energy budget of juvenile white sturgen. J Fish Biol 49:863–876
De Silva SS (1973) Food and feeding habits of the herring Clupea harengus and the sprat C. sprattus in inshore waters of the west coast of Scotland. Mar Biol 20:282–290
De Silva SS, Balbontin F (1974) Laboratory studies on food intake and food conversion of young herring, Clupea harengus (L.). J Fish Biol 6:645–658
Dickmann M, Möllmann C, Voss R (2007) Feeding ecology of Central Baltic sprat Sprattus sprattus larvae in relation to zooplankton dynamics: implications for survival. Mar Ecol Prog Ser 342:277–289
Durbin AG, Durbin EG, Verity PG, Smayda TJ (1981) Voluntary swimming speeds and respiration rates of a filter-feeding planktivore, the Atlantic menhaden, Brevoortia tyrannus (Pisces: Clupeidae). Fish Bull 78(4):877–886
Elliott JM (1975) The growth rate of brown trout (Salmo trutta L.) fed on reduced rations. J Anim Ecol 44:823–842
Engelhard GH, Peck MA, Smout S, Anna Rindorf A, Raab K, Aarts G, van Deurs M, Brunel T, Lauerburg RAM, Garthe S, Andersen KH, van Kooten T, Scott F, Dickey-Collas M (2014) Forage fish, their fisheries, and their predators: who drives whom? ICES J Mar Sci 71:90–104
Foss A, Imsland AK (2002) Compensatory growth in the spotted wolffish Anarhichas minor (Olafsen) after a period of limited oxygen supply. Aquac Res 33:1097–1101
Gerking SD (1971) Influence of rate of feeding and body weight on protein metabolism of bluegill sunfish. Physiol Zool 44:9–19
Houde ED, Schekter RC (1981) Growth rates, rations and cohort consumption of marine fish larvae in relation to prey concentrations. Rapports et Procès-verbaux des Réunions du Conseil International pour l’Exploration de la Mer 178:441–453
Houde ED, Zastrow CE (1993) Ecosystem- and taxon-specific dynamic and energetic properties of larval fish assemblages. Bull Mar Sci 53:290–335
Jobling M (1994) Fish bioenergetics. Chapman Hall, London
Jobling M, Meloy OH, dos Santos J, Christiansen B (1994) The compensatory growth response of the Atlantic cod: effects of nutritional history. Aquac Int 2:75–90
Johansson F, Leonardsson K (1998) Swimming speeds and activity levels of consumers at various resource and consumer densities under predation risk. Can J Zool 76:76–82
Jørgensen SE (1976) A eutrophication model for a lake. Ecol Model 2:147–165
Keckeis H, Schiemer F (1992) Food consumption and growth of larvae and juveniles of three cyprinid species at different food levels. Environ Biol Fish 33:33–45
Kiørboe T, Munk P (1986) Feeding and growth of larval herring, Clupea harengus, in relation to density of copepod nauplii. Environ Biol Fish 17:133–139
Kiørboe T, Munk P, Richardson K (1987) Respiration and growth of larval herring Clupea harengus: relation between specific dynamic action and growth efficiency. Mar Ecol Prog Ser 40:1–10
Köster FW, Möllmann C (2000) Trophodynamic control by clupeid predators on recruitment success in Baltic cod? ICES J Mar Sci 57:310–323
MacKenzie BR, Köster FW (2004) Fish Production and climate: sprat in the Baltic Sea. Ecology 85:784–794
Malloy KD, Targett TE (1994) Effects of ration limitation and low temperature on growth, biochemical condition, and survival of juvenile summer flounder from two Atlantic coast nurseries. Trans Am Fish Soc 123:182–193
Maar M, Rindorf A, Møller EF, Christensen A, Madsen KS, van Deurs M (2014) Zooplankton mortality in 3D ecosystem modelling considering variable spatial-temporal fish consumptions in the North Sea. Prog Oceanogr 124:78–91
Méndez G, Wieser W (1993) Metabolic responses to food deprivation and refeeding in juveniles of Rutilus rutilus (Teleostei: Cyprinidae). Environ Biol Fish 36:73–81
Miglavs I, Jobling M (1989) Effects of feeding regime on food consumption, growth rates and tissue nucleic acids in juvenile Arctic charr, Salvelinus alpinus, with particular respect to compensatory growth. J Fish Biol 34:947–957
Molony BW (1993) Effects of feeding history on mobilization and deposition of body constituents and on growth on juvenile Ambassis vachelli (Pisces: Chandidae). Mar Biol 116:389–397
Mortensen A, Damsgård B (1993) Compensatory growth and weight segregation following light and temperature manipulation of juvenile Atlantic salmon (Salmo salar L.) and Arctic charr (Salvelinus alpinus L.). Aquaculture 114:261–272
Nicieza A, Metcalfe NB (1997) Growth compensation in juvenile Atlantic salmon: responses to depressed temperature and food availability. Ecology 78:2385–2400
Opaliński KW, Maciejewska K, Krajewska-Soltys A, Fey DP (2004) Production and oxygen consumption in the early life stages of herring and smelt in Vistula Lagoon (Baltic Sea). Bull Sea Fish Inst Gdynia 2(162):13–22
Paul AJ, Paul JM, Smith RL (1995) Compensatory growth in Alaska yellowfin sole, Pleuronectes asper, following food deprivation. J Fish Biol 46:442–448
Peck MA, Baumann H, Bernreuther M, Clemmesen C, Herrmann J-P, Huwer B, Kanstinger P, Petereit C, Temming A, Voss R (2012) The ecophysiology of Sprattus sprattus in the Baltic and North Seas. Prog Oceanogr 103:42–57
Peck MA, Buckley LJ, Caldarone EM, Bengtson DA (2003a) Effects of food consumption and temperature on growth rate and biochemical-based indicators of growth in early juvenile Atlantic cod (Gadus morhua) and haddock (Melanogrammus aeglefinus). Mar Ecol Prog Ser 251:233–243
Peck MA, Clemmesen C, Herrmann J-P (2005) Ontogenic changes in the allometric scaling of the mass–length relationship in Sprattus sprattus. J Fish Biol 66:882–887
Peck MA, Katersky RS, Menard LM, Bengtson DA (2003b) The effect of body size on food consumption, absorption efficiency, respiration, and ammonia excretion by the inland silverside, Menidia beryllina (Cope) (Osteichthyes: Atherinidae). J Appl Ichthyol 19(4):195–201
Peck MA, Reglero P, Takahashi M, Catalán IA (2013) Life cycle ecophysiology of small pelagic fish and climate-driven changes in populations. Prog Oceanogr 116:220–245
Pethybridge H, Roos D, Loizeau V, Pecquerie L, Bacher C (2013) Responses of European anchovy vital rates and population growth to environmental fluctuations: an individual-based modeling approach. Ecol Model 250:370–383
Pikitch E, Boersma PD, Boyd IL, Conover DO, Cury P, Essington T, Heppell SS, Houde ED, Mangel M, Pauly D, Plagányi É, Sainsbury K, Steneck RS (2012) Little fish, big impact: managing a crucial link in ocean food webs. Lenfest Ocean Program. Washington, DC. 108 pp
R Core Team (2012) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0, URL http://www.R-project.org/
Ranney SH (2008) Influence of feeding history on metabolic rates in fishes: evidence for metabolic compensation in largemouth bass (Micropterus salmoides). Master of Science Thesis, Department of Wildlife and Fisheries Sciences, South Dakota State University, South Dakota, USA, 86 pp
Rudstram LG, Magnuson JJ (1985) Predicting the vertical distribution of fish populations: analysis of cisco, Coregonus artedii, and yellow perch, Perca flavescens. Can J Fish Aquat Sci 42:1178–1188
Russell NR, Wootton RJ (1992) Appetite and growth compensation in the European minnow, Phoxinus phoxinus (Cyprinidae) following short term of food restriction. Environ Biol Fish 34:277–285
Shields RJ (1989) Studies of growth and nutritional status in 0-group sprat, Sprattus sprattus (Clupeidae), using otolith microstructure and lipid analytical techniques. Ph.D. Thesis, University of Wales, Bangor
Sogard SM, Olla BL (1996) Food deprivation affects vertical distribution and activity of a marine fish in a thermal gradient: potential energy conserving mechanisms. Mar Ecol Prog Ser 133:43–55
Sogard SM, Olla BL (2001) Growth and behavioral responses to elevated temperatures by sablefish Anaplopoms fimbria and the interactive role of food availability. Mar Ecol Prog Ser 217:121–134
Shaw JJ, Tregenza T, Parker GA, Harvey IF (1995) Evolutionary stable foraging speeds in feeding scrambles: a model and an experimental test. Proc R Soc Lond Biol 260:273–277
van Dijk PL, Staaks G, Hardewig I (2002) The effect of fasting and refeeding on temperature preference, activity and growth of roach, Rutilus rutilus. Oecologia 130(4):496–504
Vasquez AV (1989) Energetics, trophic relationships and chemical composition of bay anchovy, Anchoa mitchilli, in the Chesapeake Bay. M.Sc. thesus, University of Maryland, College Park
Voss R, Köster FW, Dickmann M (2003) Comparing the feeding habits of co-occurring sprat (Sprattus sprattus) and cod (Gadus morhua) larvae in the Bornholm Basin, Baltic Sea. Fish Res 1497:1–15
Ware DM (1975) Growth, metabolism and optimal swimming speed of a pelagic fish. J Fish Res Board Can 32:33–41
Wieser W (1991) Limitations of energy acquisition and energy use in small poikilotherms: evolutionary implications. Funct Ecol 5:234–240
Wieser W, Krumschnabel G, Ojwang-Okwor (1992) The energetics of starvation and growth after refeeding in juveniles of three cyprinid species. Environ Biol Fish 33:63–71
Winberg GG (1956) Intensivnost obmena i pishchevye potrebnosti ryb. Nauchnye Trudy Belorusskovo Gosudarstvennovo Universiteta imeni V. I. Lenina, Minsk, pp 253
Acknowledgments
We would like to thank Hannes Baumann and Philipp Kanstinger for their help with data collection and for the field capture and rearing of sprat post-larvae. The work complies with animal health and welfare laws (TierSchG, Reg.-Nr. 3/2011 – Freie und Hansestadt Hamburg Behörde für Gesundheit und Verbraucherschutz). This research was part of the “GLOBEC-Germany” program funded by the German Federal Ministry for Education and Research (BMBF). Partial funding for this research was also received from the “FACTS” (Forage Fish Interactions, EU FP7, 244966). This research is a contribution of MAP to the Visiting Professor Program at King Saud University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Peck, M.A., Ewest, B., Herrmann, JP. et al. Relationships between feeding, growth and swimming activity of European sprat (Sprattus sprattus L.) post-larvae in the laboratory. Environ Biol Fish 98, 1117–1127 (2015). https://doi.org/10.1007/s10641-014-0345-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10641-014-0345-5