Abstract
Antibiotic effectiveness can be viewed as a biological commons since one individual's current use may decrease future effectiveness for everyone else. The value of the biological commons declines when the targeted bacteria develop antibiotic resistance. Antibiotic resistance is a global threat to health and development, causing serious economic damage and loss of human lives. The greatest share of antibiotics is used in livestock production, leading to concerns that such use may threaten human health. While various policies are in place to promote judicious use of antibiotics, their effectiveness is unclear. One key challenge in antibiotics management is the uncertainty surrounding various decisions related to antibiotic use, including whether a suspect case has an infection, how likely an infection will spread, and how effective antibiotics can be if used. We develop a disease management model that incorporates linkages among diagnostic testing decisions, antibiotic use decisions, and alternative treatment costs. We show that many unintended consequences may arise from policies designed to promote judicious antibiotic use. Antibiotics and self-tests are complements (substitutes) whenever antibiotic cost is high (low), implying that a self-test subsidy can plausibly increase expected antibiotic use. With regard to a prescription regulation (PR) that switches an antibiotic from over-the-counter to prescription, we show that while PR can reduce therapeutic antibiotic use as intended it may not achieve the social optimum. In a simple real-world application, we find that PR induces excessive veterinary service demand but does not reduce antibiotic use among typical U.S. dairy farms. PR also leads to the substitution of veterinary services for self-tests in obtaining information. We discuss how our analytical framework can be applied to other contexts, including antibiotics for human use.

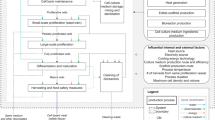
Note: (1) We underscore farmer’s decisions in infection management, i.e., test decision, veterinary service decision and antibiotic decision. (2) In a test decision, the farmer can choose a self-test to reveal information about infection types. After the self-test has revealed the information, the farmer can decide whether to call a veterinarian for veterinary services. The broken arrow connecting test decision and veterinary service decision indicates that the veterinary service decision does not necessarily occur. The veterinary service decision occurs if and only if a self-test has been chosen to reveal information

Note: Above the dashed line, nature plays first and determines the infection type, E or I. Below the dashed line, a farmer makes three linked decisions. At information set ①, the farmer decides whether to self-test (Te), call a veterinarian (C), or do neither (NTe, NC). Then at information sets ②–③ knowing the infection type the farmer decides whether to call (C) or not call (NC) a veterinarian for other services. Last, the farmer decides whether to treat (Tr) or not treat (NTr) with antibiotics at information sets ④–⑩

Note: Solid lines indicate boundary conditions under which the optimal strategy switches for farmers without any regulations

Note: Dashed lines indicate boundary conditions under which PR regulated farmer’s optimal strategy switches

Note: Solid lines indicate boundary conditions across which an unregulated farmer's optimal strategy switches. Dashed lines indicate boundary conditions across which a regulated farmer's optimal strategy switches. Each area represents the optimal strategy given the values of (d, v) either with or without the PR constraint.

Note: (1) Solid lines indicate boundary conditions across which an unregulated farmer’s optimal strategy switches. Analogously, dashed lines and dotted lines depict, respectively, boundary conditions for a PR regulated farmer’s optimal strategy and the social optimal strategy. Each area represents an optimal strategy given the values of (d, v) either with or without the PR constraint. (2) In the white areas, the unregulated farmer’s strategies correspond to the socially optimal level while PR changes the wedge between actual strategies and socially optimal strategies. In dark grey areas, PR may change sub-optimal private strategies but does not induce social optimum. In light grey area, PR improves the farmer’s strategies and produces social optimum
Similar content being viewed by others
Change history
02 May 2024
A Correction to this paper has been published: https://doi.org/10.1007/s10640-024-00876-9
Notes
All antibiotics and their associated classes listed in Appendix A of FDA’s Guidance for Industry #152 are considered to be “medically important” in human medical therapy.
This assumption allows us to focus on the issue at hand which is the nexus of decisions on antibiotic administration and information purchase and their interactions.
A detailed explanation of Fig. 3 is provided in SM 1.3.1. Detailed analyses for other cases are available on website https://yananjia.github.io/research/.
Under Assumption VI, the payoff without infections \(a\) is unnecessary in this model. This is because \(a\) is constant and so it cancels when comparing payoffs, having no effect on optimal strategies. Without assumption VI, the payoff without infections \(a\) can change accordingly and so becomes important in regulation assessment. Future research could investigate model extensions to allow for equilibrium price changes.
Detailed explanations for Fig. 4 are given in SM 2.1. We have analyzed other cases and details are available on website https://yananjia.github.io/research/.
Optimal solutions under prescription regulation are given in SM 2.
Since payoffs are \(\Phi_{E}^{Te,NC,Tr} = a - l_{1} - b - d\) and \(\Phi_{E}^{Te,NC,NTr} = a - l_{3} - d\), inequality (A.1) can be written as inequality (A.2). All possible payoffs as functions of cost parameters are laid out in SM 1.1.
Since payoffs are \(\Phi_{E}^{NTe,NC,Tr} = a - l_{1} - b\), \(\Phi_{E}^{NTe,NC,NTr} = a - l_{3}\), \(\Phi_{I}^{NTe,NC,Tr} = a - l_{3} - b\), \(\Phi_{I}^{NTe,NC,NTr} = a - l_{3}\), inequality (A.3) can be written as inequality (A.4).
Since payoffs are \(\Phi_{E}^{Te,NC,Tr} = a - l_{1} - b - d\), \(\Phi_{I}^{Te,NC,NTr} = a - l_{3} - d\), \(\Phi_{E}^{NTe,NC,Tr} = a - l_{1} - b\) and \(\Phi_{I}^{NTe,NC,Tr} = a - l_{3} - b\), inequality (A.5) is equivalent to inequality (A.6).
Since payoffs are \(\Phi_{E}^{Te,NC,Tr} = a - l_{1} - b - d\), \(\Phi_{I}^{Te,NC,NTr} = a - l_{3} - d\), and \(\Phi_{E}^{NTe,NC,NTr} = \Phi_{I}^{NTe,NC,NTr} = a - l_{3}\), inequality (A.7) is equivalent to inequality (A.8).
Since payoffs are \(\Phi_{E}^{Te,NC,NTr} = \Phi_{I}^{Te,NC,NTr} a - l_{3} - d\), \(\Phi_{E}^{NTe,NC,NTr} = \Phi_{I}^{NTe,NC,NTr} = a - l_{3}\) and \(d > 0\), inequality (A.9) does not hold.
Antibiotics are used prophylactically in procedures, such as cesarean sections, joint replacements, improved cancer treatments, and organ transplants. These procedures cannot be performed in the absence of an effective antibiotic. According to one estimate, the consequence would be an approximate 4% loss in world GDP, or a US $120 trillion loss by 2050 (Tillotson and Zinner 2017).
References
Adda J (2020) Preventing the spread of antibiotic resistance. AEA Pap Proc 110:255–259. https://doi.org/10.1257/pandp.20201014
Amer S, Gálvez FLA, Fukuda Y, Tada C, Jimenez IL, Valle WFM, Nakai Y (2018) Prevalence and etiology of mastitis in dairy cattle in El Oro province. Ecuad J Vet Med Sci 80(6):861–868. https://doi.org/10.1292/jvms.17-0504
Australian Government (2023) AMR and animal health in Australia. Retrieved from https://www.amr.gov.au/about-amr/amr-australia/animal-health
Belay DG, Abate TG, Jensen JD (2020) A montero auction mechanism to regulate antimicrobial consumption in agriculture. Am J Agr Econ 102(5):1448–1467. https://doi.org/10.1002/ajae.12079
Belay DG, Jensen JD (2021) Quantitative input restriction and farmers’ economic performance: evidence from Denmark’s yellow card initiative on antibiotics. J Agric Econ. https://doi.org/10.1111/1477-9552.12439
Cecchini M, Langer J, Slawomirski L (2015) Resistance in G7 countries and beyond: economic issues, policies and options for actions. OECD. https://www.oecd.org/els/health-systems/Antimicrobial-Resistance-in-G7-Countries-and-Beyond.pdf
Cha E, Bar D, Hertl JA, Tauer LW, Bennett G, González RN, Schukken YH, Welcome FL, Gröhn YT (2011) The cost and management of different types of clinical mastitis in dairy cows estimated by dynamic programming. J Dairy Sci 94(9):4476–4487. https://doi.org/10.3168/jds.2010-4123
Chang Q, Wang W, Regev-Yochay G, Lipsitch M, Hanage WP (2015) Antibiotics in agriculture and the risk to human health: how worried should we be? Evol Appl 8(3):240–247. https://doi.org/10.1111/eva.12185
Chatterjee A, Modarai M, Naylor NR, Boyd SE, Atun R, Barlow J, Holmes AH, Johnson A, Robotham JV (2018) Quantifying drivers of antibiotic resistance in humans: a systematic review. Lancet Infect Dis 18(12):e368–e378. https://doi.org/10.1016/S1473-3099(18)30296-2
Da Silva RA, Arenas NE, Luiza VL, Bermudez JAZ, Clarke SE (2023) Regulations on the use of antibiotics in livestock production in South America: a comparative literature analysis. Antibiotics. https://doi.org/10.3390/antibiotics12081303
Dadgostar P (2019) Antimicrobial resistance: implications and costs. Infect Drug Res 12:3903–3910. https://doi.org/10.2147/IDR.S234610
Elphinston RA, Connor JP, de Andrade D, Hipper L, Freeman C, Chan G, Sterling M (2021) Impact of a policy change restricting access to codeine on prescription opioid-related emergency department presentations: an interrupted time series analysis. Pain 162(4):1095–1103. https://doi.org/10.1097/j.pain.0000000000002115
Elyazeed HA, Al-Atfeehy NM, Abotaleb R, Sayed R, Marouf S (2020) Preparation of ELISA and lateral flow kits for rapid diagnosis of Mycoplasma gallisepticum in poultry. Sci Rep 10(1):3–8. https://doi.org/10.1038/s41598-020-65848-7
European Centre for Disease Prevention and Control [ECDC], Organisation for Economic Co-operation and Development [OECD] (2019) Antimicrobial resistance. Tackling the burden in the European Union. In briefing note for EU/EEA countries
European Medicines Agency (2020) Categorisation of antibiotics in the European Union. Retrieved from https://www.ema.europa.eu/en/documents/report/categorisation-antibiotics-european-union-answer-request-european-commission-updating-scientific_en.pdf
Feng H, Hennessy DA, Jia Y, McKendree MGS, Wolf CA (2018) Dairy sector consolidation, scale, automation and factor biased technical change: working through “get big or get out.” Choices 33(4):1–8
Gray ST (2011) From uncertainty to action: climate change projections and the management of large natural areas. Bioscience 61(7):504–505
Hayes DJ, Jensen HH (2003) Lessons from the Danish ban on feed-grade antibiotics. Briefing Paper. Center for Agricultural and Rural Development, Iowa State University, 03-BP 41, pp 12
He Y, Yuan Q, Mathieu J, Stadler L, Senehi N, Sun R, Alvarez PJJ (2020) Antibiotic resistance genes from livestock waste: occurrence, dissemination, and treatment. NPJ Clean Water. https://doi.org/10.1038/s41545-020-0051-0
Hoelzer K, Wong N, Thomas J, Talkington K, Jungman E, Coukell A (2017) Antimicrobial drug use in food-producing animals and associated human health risks: what, and how strong, is the evidence? BMC Vet Res 13(1):1–38. https://doi.org/10.1186/s12917-017-1131-3
Hollis A, Ahmed Z (2013) Preserving antibiotics, rationally. N Engl J Med 369(26):2474–2476. https://doi.org/10.1056/nejmp1312654
Kniesner TJ, Viscusi WK (2019) Legal studies research paper series: the value of a statistical life. Oxf Res Encycl Econ Financ 19:15–19
Koch BJ, Hungate BA, Price LB (2017) Food-animal production and the spread of antibiotic resistance: the role of ecology. Front Ecol Environ 15(6):309–318. https://doi.org/10.1002/fee.1505
Krömker V, Leimbach S (2017) Mastitis treatment—Reduction in antibiotic usage in dairy cows. Reprod Domest Anim 52:21–29. https://doi.org/10.1111/rda.13032
Larsson DGJ, Andremont A, Bengtsson-Palme J, Brandt KK, de Roda Husman AM, Fagerstedt P, Fick J, Flach CF, Gaze WH, Kuroda M, Kvint K, Laxminarayan R, Manaia CM, Nielsen KM, Plant L, Ploy MC, Segovia C, Simonet P, Smalla K, Snape J, Topp E, Van Hengel AJ, Verner-Jeffreys DW, Virta MPJ, Wellington EM, Wernersson AS (2018) Critical knowledge gaps and research needs related to the environmental dimensions of antibiotic resistance. Environ Int 117(March):132–138. https://doi.org/10.1016/j.envint.2018.04.041
Laxminarayan R, Brown GM (2001) Economics of antibiotic resistance: a theory of optimal use. J Environ Econ Manag 42(2):183–206. https://doi.org/10.1006/jeem.2000.1156
Laxminarayan R, Duse A, Wattal C, Zaidi AKM, Wertheim HFL, Sumpradit N, Vlieghe E, Hara GL, Gould IM, Goossens H, Greko C, So AD, Bigdeli M, Tomson G, Woodhouse W, Ombaka E, Peralta AQ, Qamar FN, Mir F, Kariuki S, Bhutta ZA, Coates A, Bergstrom R, Wright GD, Brown ED, Cars O (2013) Antibiotic resistance-the need for global solutions. Lancet Infect Dis 13(12):1057–1098. https://doi.org/10.1016/S1473-3099(13)70318-9
Laxminarayan R, Weitzman ML (2002) On the implications of endogenous resistance to medications. J Health Econ 21(4):709–718. https://doi.org/10.1016/S0167-6296(02)00034-6
Liang D, Arnold LM, Stowe CJ, Harmon RJ, Bewley JM (2017) Estimating US dairy clinical disease costs with a stochastic simulation model. J Dairy Sci 100(2):1472–1486. https://doi.org/10.3168/jds.2016-11565
Ma F, Xu S, Tang Z, Li Z, Zhang L (2021) Use of antimicrobials in food animals and impact of transmission of antimicrobial resistance on humans. Biosafety and Health 3(1):32–38. https://doi.org/10.1016/j.bsheal.2020.09.004
Marshall BM, Levy SB (2011) Food animals and antimicrobials: impacts on human health. Clin Microbiol Rev 24(4):718–733. https://doi.org/10.1128/CMR.00002-11
Mathew AG, Cissell R, Liamthong S (2007) Antibiotic resistance in bacteria associated with food animals: a United States perspective of livestock production. Foodborne Pathog Dis 4(2):115–133. https://doi.org/10.1089/fpd.2006.0066
Merriman K, Maunsell F, Nelson C, Vries A de (2014) Selective Antibiotic Treatment for Dairy Cow Mastitis. EDIS, 1–5
Mulchandani R, Wang Y, Gilbert M, Van Boeckel TP (2023) Global trends in antimicrobial use in food-producing animals: 2020 to 2030. PLOS Glob Public Health 3(2):e0001305. https://doi.org/10.1371/journal.pgph.0001305
Murray CJ, Ikuta KS, Sharara F, Swetschinski L, Robles Aguilar G, Gray A, Han C, Bisignano C, Rao P, Wool E, Johnson SC, Browne AJ, Chipeta MG, Fell F, Hackett S, Haines-Woodhouse G, Kashef Hamadani BH, Kumaran EAP, McManigal B, Naghavi M (2022) Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. The Lancet 399(10325):629–655
Mutua F, Sharma G, Grace D, Bandyopadhyay S, Shome B, Lindahl J (2020) A review of animal health and drug use practices in India, and their possible link to antimicrobial resistance. Antimicrob Res Infect Control. https://doi.org/10.1186/s13756-020-00760-3
Nair M, Zeegers MP, Varghese GM, Burza S (2021) India’s National Action Plan on antimicrobial resistance: a critical perspective. J Glob Antimicrob Res 27:236–238. https://doi.org/10.1016/j.jgar.2021.10.007
National Health Commission of the People’s Republic of China (2022) National action plan to contain antimicrobial resistance (2022–2025). Retrieved from http://www.nhc.gov.cn/yzygj/s7659/202210/2875ad7e2b2e46a2a672240ed9ee750f.shtml
O’Neill J (2016) Tackling drug-resistant infections globally: final report and recommendations. https://amr-review.org/sites/default/files/160525_Final%20paper_with%20cover.pdf
Persson Waller K, Hardemark V, Nyman AK, Duse A (2016) Veterinary treatment strategies for clinical mastitis in dairy cows in Sweden. Vet Record 178(10):240. https://doi.org/10.1136/vr.103506
Pinzón-Sánchez C, Cabrera VE, Ruegg PL (2011) Decision tree analysis of treatment strategies for mild and moderate cases of clinical mastitis occurring in early lactation. J Dairy Sci 94(4):1873–1892. https://doi.org/10.3168/jds.2010-3930
Pulingam T, Parumasivam T, Gazzali AM, Sulaiman AM, Chee JY, Lakshmanan M, Chin CF, Sudesh K (2022) Antimicrobial resistance: prevalence, economic burden, mechanisms of resistance and strategies to overcome. Eur J Pharm Sci. https://doi.org/10.1016/j.ejps.2021.106103
Redding LE, Bender J, Baker L (2019) Quantification of antibiotic use on dairy farms in Pennsylvania. J Dairy Sci 102(2):1494–1507. https://doi.org/10.3168/jds.2018-15224
Robinson TP, Bu DP, Carrique-Mas J, Fèvre EM, Gilbert M, Grace D, Hay SI, Jiwakanon J, Kakkar M, Kariuki S, Laxminarayan R, Lubroth J, Magnusson U, Ngoc PT, van Boeckel TP, Woolhouse MEJ (2016) Antibiotic resistance is the quintessential One Health issue. Trans R Soc Trop Med Hyg 110(7):377–380. https://doi.org/10.1093/trstmh/trw048
Rising J, Tedesco M, Piontek F, Stainforth DA (2022) The missing risks of climate change. Nature 610(7933):643–651. https://doi.org/10.1038/s41586-022-05243-6
Ruegg PL (2019) Responsible use of antibiotics for treatment of clinical Mastitis. Retrieved from https://dairy-cattle.extension.org/responsible-use-of-antibiotics-for-treatment-of-clinical-mastitis/
Ruegg, P. L. (2020). Understanding the economic impact of mastitis therapy. In: Proceedings of the Third Recent Graduate Conference, American Association of Bovine Practitioners, Columbus, Ohio, USA, 20–22 February 2020, vol 53(1), pp 84–91. https://doi.org/10.21423/aabppro20207976
Selvarajan R, Obize C, Sibanda T, Abia ALK, Long H (2023) Evolution and emergence of antibiotic resistance in given ecosystems: possible strategies for addressing the challenge of antibiotic resistance. Antibiotics. https://doi.org/10.3390/antibiotics12010028
Schelling TC (2007) Climate change: the uncertainties, the certainties, and what they imply about action. Econ Voice. https://doi.org/10.2202/1553-3832.1276
Schmerold I, van Geijlswijk I, Gehring R (2023) European regulations on the use of antibiotics in veterinary medicine. Eur J Pharm Sci. https://doi.org/10.1016/j.ejps.2023.106473
Shao Y, Wang Y, Yuan Y, Xie Y (2021) A systematic review on antibiotics misuse in livestock and aquaculture and regulation implications in China. Sci Total Environ. https://doi.org/10.1016/j.scitotenv.2021.149205
Sneeringer S, Bowman M, Clancy M (2019) The U.S. and EU animal pharmaceutical industries in the age of antibiotic resistance. US Department of Agriculture, Economic Research Service, No 1477–2019–2172
Swann Committee (1969) Report of the joint committee on the use of antibiotics in animal husbandry and veterinary medicine. http://books.google.ca/books?id=LKo4GwAACAAJ
Tillotson GS, Zinner SH (2017) Burden of antimicrobial resistance in an era of decreasing susceptibility. Expert Rev Anti Infect Ther 15(7):663–676. https://doi.org/10.1080/14787210.2017.1337508
U.S. Center for Disease Control and Prevention [US CDC] (2013) Antibiotic resistance threats in the United States. Retrieved from https://www.cdc.gov/drugresistance/pdf/ar-threats-2013-508.pdf.
U.S. Center for Disease Control and Prevention [US CDC] (2019) Antibiotic resistance threats in the United States. Retrieved from www.cdc.gov/DrugResistance/Biggest-Threats.html
U.S. Department of Agriculture [USDA]. (2016). Milk quality, milking procedures, and mastitis on U.S. dairies. Retrieved from https://www.aphis.usda.gov/animal_health/nahms/dairy/downloads/dairy14/Dairy14_dr_Mastitis.pdf.
U.S. Food and Drug Administration [US FDA] (2012) The judicious use of medically important antimicrobial drugs in food-producing animals. Retrieved from https://www.fda.gov/media/79140/download.
U.S. Food and Drug Administration [US FDA] (2013) Guidance for industry #213—New animal drugs and new animal drug combination products administered in or on medicated feed or drinking water of food producing animals: recommendations for drug sponsors for voluntarily aligning product use conditions with GFI #209. Retrieved from https://www.fda.gov/media/83488/download
U.S. Food and Drug Administration [US FDA] (2021a) Guidance for industry #263—Recommendations for sponsors of medically important antimicrobial drugs approved for use in animals to voluntarily bring under veterinary oversight all products that continue to be available Over-the-Counter. Center for Veterinary Medicine. Retrieved from https://www.fda.gov/media/130610/download
U.S. Food and Drug Administration [US FDA] (2021b) Summary report on antimicrobials sold or distributed for use in food-producing animals. Retrieved from https://www.fda.gov/media/154820/download.
U.S. Food and Drug Administration [US FDA] (2022) Summary Report on Antimicrobials Sold or Distributed for Use in Food-Producing Animals
Väänänen MH, Pietilä K, Airaksinen M (2006) Self-medication with antibiotics-Does it really happen in Europe? Health Policy 77(2):166–171. https://doi.org/10.1016/j.healthpol.2005.07.001
Wee BA, Muloi DM, van Bunnik BAD (2020) Quantifying the transmission of antimicrobial resistance at the human and livestock interface with genomics. Clin Microbiol Infect 26(12):1612–1616. https://doi.org/10.1016/j.cmi.2020.09.019
Wemette M, Safi AG, Beauvais W, Ceres K, Shapiro M, Moroni P, Welcome FL, Ivanek R (2020) New York State dairy farmers’ perceptions of antibiotic use and resistance: a qualitative interview study. PLoS ONE 15(5):1–23. https://doi.org/10.1371/journal.pone.0232937
World Health Organization [WHO] (2017) WHO Guidelines on use of medically important antimicrobials in food-producing animals. https://www.ncbi.nlm.nih.gov/books/NBK493702/.
World Health Organization [WHO]. (2019) Critically important antimicrobials for human medicine, In: 6th revision. Retrieved from https://apps.who.int/iris/bitstream/handle/10665/312266/9789241515528-eng.pdf
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We have no conflict of interest to declare and have no relevant or material financial interests that relate to the research described in this paper. This research was supported in part by the Elton R. Smith Endowment at Michigan State University.
Ethical Approval
We have obtained approval from all appropriate Institutional Review Boards. We are able to permanently share data and code for replication according to the Data and Documentation Policy. As Corresponding Author, I confirm that the manuscript has been read and approved for submission by all the named authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Appendices
Appendix
A1 Maximization problem in a special case: when veterinary service cost is high, \(v > l_{3} - l_{2}\)
When facing a veterinary service cost that is sufficiently high (specifically, \(v > l_{3} - l_{2}\)) to outweigh the loss reduction from veterinary services, the farmer does not prefer veterinary services. As modeled in Fig. 2 in the main text, for this setting all branches related to veterinary services are dominated. Therefore the decision tree can be simplified to be that presented in Fig.7.
Nature plays first and determines the infection types, E or I. Then at information set ① the farmer first decides whether to self-test for infection type (Te). At information set ⑥, ⑦, and ⑨ the farmer makes treatment decisions. When a self-test reveals type E at information set ⑥, the farmer makes antibiotic administration decision. When a self-test reveals type I at information set ⑨, the farmer does not use antibiotics. When no information is purchased at information set ①, then at information set ⑦ the farmer makes antibiotic administration decision.
Backward induction is used to derive optimal strategies. First, at information set ⑥ and ⑦ we solve for optimal antibiotic administration decisions that generate maximum expected payoff. Then at information set ① we solve for optimal self-testing decisions, taking the optimal antibiotic administration decisions as given.
A1.1 Optimal antibiotic decisions
At information set ⑥, a self-test reveals antibiotic to be an effective treatment for the infection at hand. The farmer administers antibiotics whenever,
or
The farmer administers antibiotics whenever antibiotic cost satisfies inequality (A.2), but not otherwise.Footnote 7
At information set ⑦, the farmer purchases no information about the antibiotic effectiveness in the infection case at hand and then makes the antibiotic administration decision based on the expected value of payoffs across infection types. The farmer administers antibiotics whenever
which can be written as
The farmer administers antibiotic whenever inequality (A.4) holds, but not otherwise.Footnote 8
We find two reservation values of antibiotic cost to be important, namely \(b_{1} = l_{3} - l_{1}\) and \(b_{2} = \beta (l_{3} - l_{1} )\). Reservation values \(b_{1}\) and \(b_{2}\) are antibiotic costs that make the farmer indifferent between Tr and NTr in scenarios where a self-test reveals type E infection and where no self-test is performed, respectively. The antibiotic cost parameter space is divided into three parts: 1) low antibiotic cost \(b < b_{2}\); 2) medium antibiotic cost \(b_{2} < b < b_{1}\); 3) high antibiotic cost \(b < b_{1}\). The optimal antibiotic administration decisions vary across antibiotic cost levels.
A1.2 Optimal self-testing decisions
When solving for optimal self-testing decisions, we take optimal antibiotic decisions at subsequent information set settings as given. Therefore we need to consider the self-testing decision at three antibiotic cost levels. At the time point when self-testing decisions are made, the farmer is uncertain about infection types. She therefore compares the expected payoffs associated with self-tests and no tests. The expected payoffs are weighted averages of payoffs in different infection cases.
A1.2.1 Low antibiotic cost \(b < b_{2}\)
The farmer chooses Tr at information sets ⑥ and ⑦. Then she makes self-testing decisions by comparing expected payoffs from self-testing and no test. Thus, the farmer performs a self-test whenever.
which can be written as
The farmer performs a self-test whenever inequality (A.6) holds but not otherwise.Footnote 9
A1.2.2 Medium antibiotic cost \(b_{2} < b < b_{1}\).
The farmer chooses Tr at information set ⑥ and NTr at information set ⑦. Then she makes self-testing decisions by comparing expected payoffs from self-testing and no test. Thus, the farmer performs a self-test whenever
which can be written as
The farmer performs a self-test if and only if this inequality holds. Footnote 10
A1.2.3 High antibiotic cost \(b > b_{1}\).
The farmer chooses NTr at information sets ⑥ and ⑦. Then she makes self-testing decisions by comparing expected payoffs from self-testing and no test. Thus, the farmer performs a self-test whenever.
As antibiotic cost has a positive value, inequality (A.9) does not hold. Therefore the farmer chooses not to perform a self-test.Footnote 11
Three optimal strategies can arise under high veterinary service cost, \(v > l_{3} - l_{2}\). They are:
S1 Do not perform a self-test at information set ①, and treat with antibiotics at information set ⑦.
S2 Perform a self-test at information set ①. In type E infection cases treat with antibiotics (at information set ⑥) while in type I infection cases do not treat with antibiotics (at information set ⑨)
S3 Do not perform a self-test at information set ①, and do not treat with antibiotics at information set ⑦.
We summarize and organize the conditions on cost parameters under which each strategy is optimal. The respective conditions under which S1-S3 are optimal are
Figure 3 in the main text presents the optimal strategies in cost parameter b-d plane, by holding veterinary service cost \(v\) fixed at a high level (i.e., \(v > l_{3} - l_{2}\)). Boundary condition \(d = b(1 - \beta )\) is depicted as the upward sloping line, boundary condition \(d = \beta (l_{3} - l_{1} - b)\) is depicted as the downward sloping line, and boundary condition \(b = \beta (l_{3} - l_{1} )\) is depicted as the vertical line. Note that the three boundary lines intersect at the same point \((\beta (l_{3} - l_{2} ),\beta (1 - \beta )(l_{3} - l_{2} ))\) in the b-d plane. In the main text we give a more detailed explanation of Fig. 3.
A2 Dairy survey data statistics
During the summer of 2017, a stratified design survey was circulated to dairy producers. Feng et al. (2018) provide some details on survey design. The response rate was 17% and a total of 660 useable surveys were received, accounting for about 4% of all registered dairy herds in these states. The survey consisted of three parts. Parts I and II of the survey inquired about farm resources, farmer demographics, overall views on the milk production business environment, and farmer expansion/contraction plans for the next three years. Antibiotic use and management behaviors, as well as perceived advantages and costs, were all investigated in Part III. Table
4 summarizes producer-level data on costs due to a mastitis case.
A3 Empirically parameterized model
We extrapolate possible values for parameters in our model from the survey data and the available literature. Table
5 summarizes parameter values in a baseline scenario and scenarios where an increase or a decrease of 20% in parameters occurs.
For self-test cost, we use the median diagnosis cost in our dataset as the baseline level, i.e., \(d = \$ 5\). This is also a reasonable value compared with estimates in the literature ($6 in Pinzón-Sánchez et al. (2011); $10 in Cha et al. (2011)). We asked about therapeutics cost in the dairy survey where therapeutics cost can include antibiotic cost but also some other veterinary drugs as well. The median therapeutics cost in our dataset is $30 and is higher than the antibiotic cost estimated in literature ($7–$25 in Ruegg (2020); $4–$8 in Cha et al. (2011); $6.75 in Pinzón-Sánchez et al. (2011)). Therefore we infer from the survey dataset and literature that \(b = \$ 10\) is a reasonable antibiotic cost. To be consistent with our assumptions, the veterinary service cost estimate should include hourly rates paid for veterinarians and costs associated with alternative treatment. The median veterinary service cost is $12 in the dataset. This is viewed as a reasonable number given that U.S. Department of Agriculture (2016) estimates veterinary service cost associated with a mastitis case on dairy farms to be in the $1.45–$9.21 range. Cha et al. (2011) estimate the cost of treatment other than antibiotics to be $15.5. We combine veterinary service cost ($12) and other treatment costs ($15.5) and assume veterinary service cost parameter d to be $27.5. This estimate is comparable to the numbers provided ($19.16 ± 15.27) in Liang et al. (2017).
Parameter \(\beta\) indicates the probability that mastitis occurs for which antibiotic treatment is effective. For most cases, antibiotics should be used to treat mastitis caused by gram-positive pathogens, while farmers should avoid antibiotic use for mastitis caused by gram-negative pathogens or when no pathogens are recovered. The incidence of mastitis caused by gram-positive pathogens is assumed to be 35% (Pinzón-Sánchez et al. 2011).
Labor and non-saleable milk costs are inevitable for a mastitis case even when antibiotics cure the mastitis case, while other losses such as death loss, loss from future milk production, loss from premature culling and loss from future reproduction are more likely to occur when the mastitis case is not treated effectively. Therefore we use the sum of median labor cost ($15) and median non-saleable milk cost ($80) to parameterize \(l_{1}\) while we add all costs and losses incurred in a mastitis case to parameterize \(l_{3}\). Median total cost is $630. Logic requires that \(l_{2}\) be greater than \(l_{1}\) but less than \(l_{3}\). When we parameterize \(l_{2}\), we should ensure it satisfies the assumption we made in Sect. 3.1 in the main manuscript, i.e., \(l_{2} - l_{1} < \beta (l_{3} - l_{1} )\). Thus, \(l_{2} \in (\$ 95,{\kern 1pt} \,{\kern 1pt} \$ 282.25)\). We assume \(l_{2} = \$ 150\) as a baseline level. Economic losses assumed are comparable to estimates in the literature (Cha et al. 2011; Liang et al. 2017; Ruegg 2020).
While many agree that antibiotic use in agriculture is a critical contributor to antibiotic resistance (Ma et al. 2021), the magnitude of its threat to human health has been insufficiently investigated (Chang et al. 2015; Dadgostar 2019; He et al. 2020). Lacking conclusive estimates in previous literature, we develop informed speculative estimates of the resistance cost that may be attributed to agricultural antibiotic use.
The estimation proceeds in steps. First we estimate total cost of antibiotic resistance in the United States, where such cost measurements are widely viewed as being both country-specific and difficult to arrive at (Murray et al. 2022; Pulingam et al. 2022; Tillotson and Zinner 2017). Our estimate consists of three parts: death due to antibiotic resistance, losses of extra health care cost incurred, and economic losses due to lost productivity. Antibiotic resistance causes 35,900 deaths, $20 billion (in 2008 dollars) in extra health care costs and $35 billion economic losses due to lost productivity annually (US CDC 2013). To place a monetary value on death caused by antibiotic resistance, we multiply the number of deaths, 35,900, by the value of a statistical life estimate $11.1 million (in 2015 dollars) (Kniesner and Viscusi 2019; US CDC 2019). Before summing up the three components of antibiotic resistance cost, we need to use an inflation factor to adjust the values of economic losses from 2008 to 2015. In 2009–2015, the U.S. inflation rate was − 0.36%, 1.64%, 3.16%, 2.07%, 1.46%, 1.62%, 0.12% respectively. We convert the inflation rate into a factor by adding 1 to each rate and then multiply these factors to get the cumulative inflation factor which is calculated to be 1.1. Then the values of economic losses in 2008 dollars multiplied by this cumulative inflation factor are the values of economic losses in 2015 dollars. Therefore, total cost is (20 + 35)*1.1 (inflation factor) + 11.1*35.9 = $459 billion (in 2015 dollars). Our total cost estimate is very likely to be downward biased because we do not include secondary external social costFootnote 12 induced by resistance (Dadgostar 2019; Tillotson and Zinner 2017).
The next step is to estimate how much agricultural antibiotic use contributes to resistance cost. Since previous quantitative research findings provide no reliable estimates of resistance cost attributable to agriculture (Chang et al. 2015), we tentatively assume that 5% of the total cost can be attributed to antibiotic use in livestock production. This number is arrived at by considering the following statements that are generally valid for the United States. The livestock farming community is not spatially well connected with major population bases. Also large livestock farms follow biosecurity protocols intent on preventing the spread of bacteria into and out of farms. Lastly, waste and runoff from livestock farms is generally contained and filtered by soils. Therefore we assume a relatively low antibiotic resistance cost as a result of on-farm antibiotic use. Total sales of antibiotics in livestock production in 2020 was 10,449,476 kg where MI antibiotics account for 57% of total sales in that year (US FDA 2021b). Assuming that the impact of MI antibiotics and N-MI antibiotics on resistance development are homogenous, we estimate the antibiotic resistance cost associated with 1 kg MI antibiotic use in animals to be $2,196. This figure is calculated as 459*109*5%/10,449,476. Assuming that antibiotic resistance arising from N-MI antibiotic use can be ignored, we estimate the antibiotic resistance cost associated with 1 kg MI antibiotic use in animals to be $3,853. This figure is calculated as 459*109*5%/(10,449,476*57%). We infer that the amount of antibiotic use in a mastitis case is about 1 g (Ruegg 2020). Therefore antibiotic resistance cost associated with antibiotic use in one mastitis case is estimated to be in the $2.2–$3.9 range.
Using these parameter values, we can indicate which scenario is most likely relevant to practices on U.S. dairy farms. We find that it is optimal for an unregulated farmer to self-test to obtain information. For revealed type E infection farmers administer OTC antibiotics while for revealed type I infection they call a veterinarian but do not use antibiotics. PR does not decrease antibiotic use but instead substitutes veterinary services for self-tests in obtaining information. Dairy farmers’ optimal strategy without regulations attains the social optimum. Therefore, PR causes excessive demand for veterinary services but does not decrease antibiotic use among typical dairy farmers.
In order to perform robustness checks, we also examine how an increase or decrease of 20% in each of these parameters affects the impact of PR. Table 5 presents the values of parameters used for robustness checks. The findings are robust when we increase or decrease parameter values by 20%.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jia, Y., Hennessy, D. & Feng, H. Economics of Informed Antibiotic Management and Judicious Use Policies in Animal Agriculture. Environ Resource Econ (2024). https://doi.org/10.1007/s10640-024-00862-1
Accepted:
Published:
DOI: https://doi.org/10.1007/s10640-024-00862-1
Keywords
- Antibiotic resistance
- Biological commons
- Information good
- Unintended distortions
- Precautionary demand
- Prescription regulation