Abstract
Background
Platinum-based doublet chemotherapy is commonly used in the treatment of non-small cell lung cancer (NSCLC). A growing body of evidence indicates that incorporating antiangiogenic agents into platinum-based chemotherapy may enhance the survival outcomes for NSCLC patients. However, the optimal administration protocol for intravenous recombinant human endostatin (rh-endostatin), an antiangiogenic agent, remains uncertain at present.
Aim
This study aims to investigate the efficacy and safety of 5-d continuous intravenous infusion of rh-endostatin in combination with chemotherapy for patients with advanced NSCLC. The predictive biomarkers for this treatment regimen were further probed.
Methods
This prospective, single-arm multicenter study enrolled a total of 48 patients with advanced NSCLC who were histologically or cytologically confirmed but had not received any prior treatment from January 2021 to December 2022. Prior to the chemotherapy, these patients received a continuous intravenous infusion of rh-endostatin (210 mg) over a period of 120 h, using an infusion pump. The chemotherapy regimen included a combination of platinum with either pemetrexed or paclitaxel, given in 21-day cycles. The primary endpoint of the study was median progression-free survival (mPFS), and the secondary endpoints included median overall survival (mOS), objective response rate (ORR), disease control rate (DCR), and assessment of adverse events (AEs).
Results
The mPFS was 6.5 months (95% confidence interval (CI): 3.8–9.1 m) while the mOS was 12.3 months (95% CI: 7.6–18.5 m). The ORR and DCR was 52.1% and 75.0%, respectively. Leukopenia (52.1%), anemia (33.3%), and thrombocytopenia (20.8%) were the most common adverse effects and these toxicities were deemed acceptable and manageable. In addition, a correlation was noted between elevated serum carcinoembryonic antigen (CEA) levels and decreased PFS and OS.
Conclusions
The incorporation of a 5-day continuous intravenous infusion of rh-endostatin into platinum-based doublet chemotherapy has demonstrated both safety and efficacy in the treatment of advanced NSCLC. Furthermore, the baseline serum levels of CEA may potentially function as a predictor for the efficacy of rh-endostatin when combined with chemotherapy in NSCLC patients.
ClinicalTrials.gov
NCT05574998.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lung cancer is the most frequent cause of cancer-related death worldwide [1]. Non-small-cell lung cancer (NSCLC) accounts for approximately 85% of lung cancers, and a significant proportion of patients are diagnosed at an advanced stage (stage IIIB/IIIC/IV) with unfavorable prognosis. The five-year survival rates range from 4 to 17%, depending on the stage and the histologic type [2]. Although the introduction of immune checkpoint inhibitors (ICIs) alone or in combination with chemotherapy has expanded treatment options for NSCLC patients [3], platinum-based doublet chemotherapy remains the primary first-line therapy for patients with advanced NSCLC lacking driver gene mutations [4,5,6]. Nevertheless, the overall benefit of platinum-based chemotherapy for advanced NSCLC is limited [7].
Angiogenesis is a complex process that plays an important role in tumor growth and metastasis. Antiangiogenic treatment promotes normalization of tumor blood vessels, enabling effective delivery of chemotherapy drugs to cancer cells [8]. Bevacizumab, the first FDA-approved angiogenesis inhibitor for advanced NSCLC, is included in the NCCN guidelines for platinum-based protocols in advanced NSCLC [9]. In a large phase III clinical trial (ECOG 4599), the combination of chemotherapy and bevacizumab demonstrated improved progression-free survival (PFS) compared to chemotherapy alone in patients with advanced NSCLC [10]. Similar results were observed in the phase III BEYOND trial involving Chinese patients [11]. However, the use of bevacizumab is limited to patients with non-squamous NSCLC and may be associated with adverse effects such as hypertension, renal toxicities and thrombosis [12, 13].
Recombinant human endostatin (Rh-endostatin), an anti-angiogenic drug developed in China, has received approval for the treatment of advanced NSCLC patients [14]. Several clinical trials have revealed that chemotherapy plus rh-endostatin can effectively treat advanced NSCLC. For example, a phase III clinical trial investigating the use of rh-endostatin in combination with chemotherapy demonstrated improved overall survival (OS), with a mOS of 13.75 months compared to 9.77 months for chemotherapy alone in NSCLC patients [15]. Another study conducted by Han et al. reported that NSCLC patients treated with platinum-based chemotherapy plus rh-endostatin achieved a higher objective response rate (ORR) with a favorable safety profile [16].
Traditionally, rh-endostatin is administered through intermittent intravenous infusion of 7.5 mg/m2 once a day for 14 days. Continuous intravenous infusion of rh-endostatin using an infusion pump has been implemented in the treatment of NSCLC in China, but consensus on the optimal infusion scheme has not been reached [17]. Pu et al. reported that the combination of rh-endostatin, chemotherapy, and camrelizumab, an anti-PD-1 antibody, demonstrated a favorable efficacy and safety profile in patients with advanced NSCLC [18]. Additionally, a study utilizing 7-day rh-endostatin regimen showed good safety in the treatment of liver metastasis from gastric cancer [19]. Qin et al. conducted a retrospective study on the continuous intravenous infusion of rh-endostatin for 5 days in combination with chemotherapy in patients with recurrent advanced NSCLC. The results demonstrated improved adherence to the 5-day continuous intravenous infusion of rh-endostatin, as well as favorable efficacy and safety [20]. Based on the theory of the vascular normalization window, the optimal timing for the use of rh-endostatin is suggested to be 4–6 days prior to chemotherapy. From this perspective, a 5-day dosing regimen of rh-endostatin seems to be feasible [21, 22]. However, there is a lack of prospective studies on the 5-day dosing regimen in combination with chemotherapy as a first-line treatment for advanced NSCLC. Therefore, the purpose of this prospective study is to evaluate the efficacy and safety of a 5-day continuous intravenous infusion of rh-endostatin plus chemotherapy in patients with advanced NSCLC.
Materials and methods
Patients
Between January 2021 and December 2022, a total of 48 treatment-naive patients with histologically or cytologically confirmed stage IV advanced NSCLC were enrolled in this study. The inclusion criteria were as follows: (1) patients aged between 18 and 75 years old; (2) Eastern Cooperative Oncology Group (ECOG) performance status score of 0 to 2; (3) expected survival of at least 3 months; (4) presence of at least one measurable disease based on the Solid Tumors Response assessment Criteria (RECIST version 1.1) [23]; (5) absence of EGFR/ALK/ROS-1 alterations; (6) no prior radiotherapy or chemotherapy; (7) sufficient blood system parameters, including hemoglobin ≥ 90 g/L, absolute neutrophil count (ANC) ≥ 1.5 × 10^9/L, and platelet count ≥ 90 × 10^9/L; (8) adequate liver and kidney function, with serum bilirubin ≤ 1.5 × upper limit of normal (ULN), alanine aminotransferase and aspartate transaminase ≤ 2.5 × ULN, alkaline phosphatase ≤ 5 × ULN, and serum creatinine 60 mL/min or higher, or creatinine clearance within the normal range. Exclusion criteria included: (1) presence of cardiopulmonary diseases such as congestive heart failure, treatment-resistant hypertension, significant arrhythmia, or recent myocardial infarction; (2) active and serious infections; (3) coagulation disorders or bleeding tendencies; (4) poor compliance. The study was reviewed and approved by the ethics committee of The First Affiliated Hospital of Shandong First Medical University, and all 48 patients provided informed consent and were able to actively participate in the treatment.
Intervention measures
In this study, patients received a continuous intravenous infusion of rh-endostatin at a dose of 210 mg over a period of 120 consecutive hours using an infusion pump. Each treatment cycle lasted for 21 days (q21d). The chemotherapy regimens varied based on the histological subtype of the NSCLC.
For patients with adenocarcinoma, the chemotherapy regimen consisted of carboplatin with an area under the receiver operating characteristic curve (AUC) of 5–6 (or cisplatin at a dose of 75 mg/m2) on day 4, in combination with pemetrexed at a dose of 500 mg on day 4, given every 21 days for a total of 4 cycles.
For patients with squamous cell carcinoma, the chemotherapy regimen included carboplatin with an AUC of 5–6 (or cisplatin at a dose of 75 mg/m2) on day 4, along with taxol at a dose of 175 mg/m2 on day 4, given every 21 days for a total of 4 cycles.
Baseline and follow-up assessment
Prior to initiating treatment, patients underwent a comprehensive set of physical, laboratory, and radiological examinations as per the study protocol. Laboratory tests included routine blood tests, coagulation function assessments, electrolyte levels, liver function tests, renal function evaluations, and the measurement of tumor markers such as neutrophil to lymphocyte ratio (NLR), carcinoembryonic antigen (CEA), neuron specific enolase (NSE) and lactate dehydrogenase (LDH). For radiological evaluation, it was performed at the beginning of treatment and every 6 weeks after the start of treatment until disease progression.
Clinical efficacy and safety evaluation
The primary endpoint was mPFS. The secondary endpoints included mOS, ORR, disease control rate (DCR), and adverse events (AEs). The therapeutic efficacy of the treatment was evaluated based on the RECIST 1.1 criteria, which classified the treatment response as complete response (CR), partial response (PR), stable disease (SD), or progressive disease (PD). The ORR was determined by tallying the number of cases with CR and PR, and subsequently dividing this total by the overall count of evaluable patients. AEs were monitored and graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE version 4.0).
Statistical analysis
All data were analyzed using GraphPad Prism version 9.0. mPFS and mOS were estimated by the Kaplan-Meier method, which allowed for the calculation of survival probabilities and construction of corresponding 95% confidence intervals (95% CI). Survival curves were generated using the Kaplan-Meier method. “Survival” and “surveyors” R packages were used to determine the cut off values for NLR, CEA, NSE, and LDH.
Results
General information
A total of 55 patients with advanced NSCLC were screened and 48 patients were assigned to receive rh-endostatin plus chemotherapy between January 2021 and December 2022 in The First Affiliated Hospital of Shandong First Medical University. Seven patients were excluded from 55 screened patients for not meeting the inclusion criteria. As a result, a total of 48 patients were included in the final analysis (Fig. 1).
Table 1 presents the baseline characteristics of the patients. The median age of the patients was 66 years old, ranging from 47 to 75 years old. Among the participants, 30 were male and 18 were female. Additionally, 7 patients (14.6%) had squamous cell carcinoma, while 41 (85.4%) patients had adenocarcinoma.
Clinical efficacy
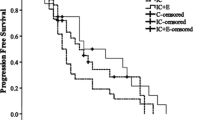
Out of the 48 patients, 25 showed a PR, 11 demonstrated SD, and 12 experienced PD (Table 2). The ORR was 52.1% (25/48) and the DCR was 75.0% (36/48). There was no significant difference in the ORR and DCR between the patients who received taxol combined with platinum and those who received pemetrexed combined with platinum (P > 0.05). The study’s final follow-up was conducted on January 31, 2023. As shown in Figs. 2 and 3, the mPFS was 6.5 months (95% CI: 3.8–9.1 m) and the mOS was 12.3 months (95% CI: 7.6–18.5 m).
Toxic effects
All 48 patients were included in the toxicity analysis. Table 3 lists the incidence of each adverse event. The majority of treatment-related adverse events were grade 1 or 2, including leukopenia (52.1%), anemia (33.3%), thrombocytopenia (20.8%), hypertension (4.2%), nausea (6.3%), vomiting (4.2%), and infection (2.1%). Major grade 3–4 adverse events were leukopenia (6.3%), anemia (2.1%), and thrombocytopenia (2.1%). Overall, the toxicities associated with rh-endostatin combined with chemotherapy in this study were deemed acceptable and manageable.
Biomarker analysis
Further investigation was conducted to explore the relationship between serum biomarker levels and patient prognosis when combining rh-endostatin with chemotherapy for NSCLC. The results uncovered a significant correlation between elevated serum CEA levels and shorter mPFS and mOS. However, no substantial correlation was observed between serum levels of NLR, NSE, LDH, and patient prognosis.
During the analysis of patients’PFS and OS, a cut-off value of 5.5 ng/ml was established for serum CEA levels. Patients with CEA levels exceeding this cut-off were classified as the high CEA group, while the remaining patients were categorized as the low CEA group. The survival curves displayed a median PFS of 4.1 months for the high CEA group and 13.0 months for the low CEA group (p = 0.01, HR = 0.32, 95% CI: 0.10–0.99) (Fig. 4). Furthermore, the analysis of survival curves demonstrated that patients in the high CEA group had a significantly shorter median OS of 10.5 months in contrast to the 16.8 months observed in the low CEA group (p = 0.04, HR = 0.63, 95% CI: 0.19–2.08) (Fig. 5).
These findings suggest that initial serum CEA levels have the potential to serve as a promising biomarker for predicting the effectiveness of combining rh-endostatin with chemotherapy in NSCLC. Elevated serum CEA levels were correlated with shorter survival durations and unfavorable prognosis. This implies that evaluating serum CEA levels could be a valuable tool in guiding treatment strategies and forecasting prognosis for NSCLC patients undergoing this combined therapy.
Discussion
Previous studies have provided evidence supporting the effectiveness of combining rh-endostatin with chemotherapy in improving PFS and OS in patients with advanced NSCLC [15, 20]. However, there is limited data available on the use of a 5-day intravenous infusion of rh-endostatin in combination with chemotherapy as a first-line treatment for advanced NSCLC. Therefore, this prospective study aimed to optimize the dosing regimen of rh-endostatin for previously untreated advanced NSCLC patients and evaluate the efficacy and safety of this treatment approach when combined with chemotherapy. The studys findings illustrated that the mPFS and mOS of NSCLC patients receiving this treatment regimen were enhanced. In addition, the toxicities were deemed acceptable and manageable. Overall, these findings add to the expanding body of evidence that supports the effectiveness and safety of combining a 5-day continuous intravenous infusion of rh-endostatin with chemotherapy as a viable first-line treatment for advanced NSCLC patients. To the best of our knowledge, this is the first prospective study investigating the use of a 5-day intravenous infusion of rh-endostatin in combination with chemotherapy as a first-line treatment for advanced driver gene-negative NSCLC.
In the current clinical practice, a combination therapy of anti-programmed cell death 1 (PD-1)/programmed cell death ligand 1 (PD-L1) antibodies and platinum-based chemotherapy has emerged as a widely employed first-line treatment for patients with advanced NSCLC that is unresectable and lacks driver mutations [24, 25]. In March 2019, the National Medical Products Administration (NMPA) of China approved the use of Opdivo and Keytruda in combination with chemotherapy as the first-line treatment for metastatic nonsquamous NSCLC in patients without EGFR and ALK mutations. However, due to the accessibility and expense of PD-1 antibodies, they were not included in our study. According to the IMpower150 study, the addition of atezolizumab to bevacizumab plus chemotherapy showed a significant improvement in PFS and OS among patients with metastatic nonsquamous NSCLC, regardless of their PD-L1 expression and EGFR or ALK genetic alteration status [26]. Therefore, our study offers an alternative treatment approach that combines PD1/PD-L1 inhibitor therapy for NSCLC patients without driver mutations, particularly for those with squamous NSCLC.
The results of this study demonstrated an ORR of 52.1% and a DCR of 75.0%. These findings are consistent with a study conducted by Sun et al., which examined the use of rh-endostatin combined with NP (vinorelbine + cisplatin) in NSCLC patients for the first time. They reported a response rate of 40% and a clinical benefit rate of 76.52% [15]. In a study by Cheng et al., the ORR and DCR for the continuous intravenous infusion of rh-endostatin in combination with first-line chemotherapy were 40.0% and 65.0%, respectively [27]. Furthermore, results from a large phase II clinical trial led by Han et al. revealed an ORR of 39.3% and a DCR of 90.2% in patients treated with rh-endostatin and paclitaxel-carboplatin (TC) [16]. In terms of prognosis and survival, Sun et al. reported a median time to progression of 6.3 months and a mOS of 13.75 months [15]. Han et al. reported that the mPFS and mOS were 7.1 versus 6.3 months (p = 0.522), and 17.6 versus 15.8 months (p = 0.696) in the treatment (TC + rh-endostatin) and control groups (TC + placebo), respectively [16]. Another study comparing different administration methods of rh-endostatin in NSCLC treatment demonstrated a mPFS of 6.0 months for patients receiving continuous intravenous infusion of rh-endostatin [27]. These results are in line with our findings in the present study.
Continuous intravenous infusion of rh-endostatin has been shown to extend the drug’s circulation time in the bloodstream and enhance its anti-tumor activity [28]. The use of portable infusion pumps for 120 h allows for continuous administration, ensuring that the anti-angiogenic treatment improves the accessibility of chemotherapy drugs to tumor cells [22]. In a study by Hansma et al., the safety of different doses of rh-endostatin was compared. It was found that a continuous intravenous infusion of rh-endostatin (210 mg) for 120 h was well-tolerated and related to the optimal time window for vascular normalization [22]. Furthermore, a study compared the efficacy and safety of continuous versus intermittent intravenous infusion of rh-endostatin in combination with chemotherapy for advanced NSCLC. The results revealed similar PFS and ORR between continuous and intermittent intravenous infusion of rh-endostatin (210 mg) [27]. Based on these findings, it can be concluded that a continuous intravenous infusion of rh-endostatin at a dose of 210 mg for 120 h is a feasible treatment regimen.
The combination of rh-endostatin and chemotherapy can result in common adverse reactions such as leukopenia, anemia, decreased appetite and hypertension [29]. In this study, grade 3–4 adverse reactions mainly consisted of leukopenia(6.3%), anemia(2.1%), and thrombocytopenia(2.1%) which were primarily related to chemotherapy. Similar grade 3–4 adverse reactions, including granulocytopenia (25.0%), anemia (5.0%), and thrombocytopenia (10.0%), were the most common in the study by Cheng et al. [27]. A meta-analysis indicated that angiogenesis inhibitors can potentially result in adverse reactions such as hypertension and myocardial ischemia [30], however the above adverse reactions were not observed in our study. Overall, the adverse events reported in the present study were manageable.
Rh-endostatin primarily targets the blood vessels of tumors rather than the tumor cells themselves. As a result, responsive patients may not experience immediate changes in tumor size following drug administration [31]. Identifying patients who will benefit from rh-endostatin is of utmost importance. Currently, there are no accurate predictors available to forecast the efficacy of rh-endostatin, although certain serum markers such as CEA and LDH have been proven to predict the efficacy of bevacizumab [32,33,34]. Gerald et al. confirmed that a high baseline CEA serum level predicted a poor outcome in patients with advanced colorectal cancer treated with bevacizumab [32]. In our study, we found that a low baseline CEA level was strongly associated with a longer PFS in NSCLC patients treated with rh-endostatin and chemotherapy. This finding is significant as it suggests that CEA levels could potentially serve as an outcome measure marker for rh-endostatin treatment in NSCLC patients. The possible reason behind this association is that overexpression of CEA enhances tumor angiogenesis. CEA induces pro-angiogenic behaviors in endothelial cells, including adhesion, spreading, proliferation, and migration in vitro, as well as tumor microvascularization in vivo independently of the vascular endothelial growth factor (VEGF)/VEGF receptor (VEGFR) system [35].
While this study provided valuable findings, it does have limitations. Firstly, the sample size was relatively small, although it was deemed sufficient to establish the conclusions of this study. However, larger sample sizes in future studies would provide more robust evidence. Secondly, this study was a single-arm study and did not include a control group for comparison. While comparing results with previous studies can offer some support for our conclusions, it is necessary to conduct controlled trials with larger sample sizes to validate our findings. Addressing these limitations through larger controlled trials would further strengthen the reliability and generalizability of the conclusions drawn from this study.
Conclusions
In conclusion, our study findings indicate that the combination of a 5-day continuous intravenous infusion of rh-endostatin and chemotherapy is both effective and safe, providing a favorable treatment option for patients with advanced NSCLC. Furthermore, the baseline serum CEA levels show promise as a potential biomarker for predicting the efficacy of rh-endostatin combined with chemotherapy in this patient population. Our study provides an alternative choice for first-line treatment of driver gene-negative advanced NSCLC. Further research and validation in larger cohorts are necessary to firmly establish the clinical utility of CEA as a predictive biomarker for this treatment regimen.
Data availability
No datasets were generated or analysed during the current study.
References
Siegel RL, Miller KD, Jemal A (2020) Cancer statistics, 2020. Cancer J Clin 70(1):7–30
Hirsch FR, Scagliotti GV, Mulshine JL et al (2017) Lung cancer: current therapies and new targeted treatments. Lancet (London England) 389(10066):299–311
Brueckl WM, Ficker JH, Zeitler G (2020) Clinically relevant prognostic and predictive markers for immune-checkpoint-inhibitor (ICI) therapy in non-small cell lung cancer (NSCLC). BMC Cancer 20(1):1185
Hanna N, Johnson D, Temin S et al (2017) Systemic therapy for Stage IV Non-small-cell Lung Cancer: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncology: Official J Am Soc Clin Oncol 35(30):3484–3515
Planchard D, Popat S, Kerr K et al (2018) Metastatic non-small cell lung cancer: ESMO Clinical Practice guidelines for diagnosis, treatment and follow-up. Annals Oncology: Official J Eur Soc Med Oncol 29(Suppl 4):iv192–iv237
Schiller JH, Harrington D, Belani CP et al (2002) Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med 346(2):92–98
Gadgeel SM, Stevenson JP, Langer CJ et al (2018) Pembrolizumab and platinum-based chemotherapy as first-line therapy for advanced non-small-cell lung cancer: phase 1 cohorts from the KEYNOTE-021 study. 125:273–281 Lung cancer (Amsterdam, Netherlands)
Goel S, Duda DG, Xu L et al (2011) Normalization of the vasculature for treatment of cancer and other diseases. Physiol Rev 91(3):1071–1121
Ettinger DS, Wood DE, Aisner DL et al (2021) NCCN guidelines Insights: Non-small Cell Lung Cancer, Version 2.2021. J Natl Compr Cancer Network: JNCCN 19(3):254–266
Sandler A, Gray R, Perry MC et al (2006) Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med 355(24):2542–2550
Zhou C, Wu YL, Chen G et al (2015) BEYOND: a Randomized, Double-Blind, Placebo-Controlled, Multicenter, Phase III Study of First-Line Carboplatin/Paclitaxel plus Bevacizumab or placebo in Chinese patients with Advanced or Recurrent Nonsquamous Non-small-cell Lung Cancer. J Clin Oncology: Official J Am Soc Clin Oncol 33(19):2197–2204
Manzo A, Montanino A, Carillio G et al (2017) Angiogenesis inhibitors in NSCLC. Int J Mol Sci.;18(10)
Reck M, von Pawel J, Zatloukal P et al (2009) Phase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first-line therapy for nonsquamous non-small-cell lung cancer: AVAil. J Clin Oncology: Official J Am Soc Clin Oncol 27(8):1227–1234
Ling Y, Yang Y, Lu N et al (2007) Endostar, a novel recombinant human endostatin, exerts antiangiogenic effect via blocking VEGF-induced tyrosine phosphorylation of KDR/Flk-1 of endothelial cells. Biochem Biophys Res Commun 361(1):79–84
Sun Y, Wang JW, Liu YY et al (2013) Long-term results of a randomized, double-blind, and placebo-controlled phase III trial: Endostar (rh-endostatin) versus placebo in combination with vinorelbine and cisplatin in advanced non-small cell lung cancer. Thorac cancer 4(4):440–448
Han B, Xiu Q, Wang H et al (2011) A multicenter, randomized, double-blind, placebo-controlled study to evaluate the efficacy of paclitaxel-carboplatin alone or with endostar for advanced non-small cell lung cancer. J Thorac Oncology: Official Publication Int Association Study Lung Cancer 6(6):1104–1109
Hu W, Fang J, Nie J et al (2016) Efficacy and safety of extended use of platinum-based doublet chemotherapy plus endostatin in patients with advanced nonsmall cell lung cancer. Medicine 95(28):e4183
Pu X, Wang Q, Liu L et al (2023) Rh-endostatin plus camrelizumab and chemotherapy in first-line treatment of advanced non-small cell lung cancer: a multicenter retrospective study. Cancer Med 12(7):7724–7733
Yang H, Sui Y, Guo X et al (2018) Endostar continuous intravenous infusion combined with S-1 and oxaliplatin chemotherapy could be effective in treating liver metastasis from gastric cancer. J Cancer Res Ther 14(Supplement):S1148–s51
Qin ZQ, Yang SF, Chen Y et al (2022) Continuous intravenous infusion of recombinant human endostatin using infusion pump plus chemotherapy in non-small cell lung cancer. World J Clin Cases 10(4):1164–1171
Jain RK (2001) Normalizing tumor vasculature with anti-angiogenic therapy: a new paradigm for combination therapy. Nat Med 7(9):987–989
He L, Zhao C, Li Y et al (2018) Antiangiogenic effects of recombinant human endostatin in lung cancers. Mol Med Rep 17(1):79–86
Eisenhauer EA, Therasse P, Bogaerts J et al (1990) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). European journal of cancer (Oxford, England: 2009;45(2):228– 47
Hayashi H, Nakagawa K (2020) Combination therapy with PD-1 or PD-L1 inhibitors for cancer. Int J Clin Oncol 25(5):818–830
Hirabayashi T, Sonehara K, Ozawa R et al (2024) Prognostic Value of the Geriatric Nutritional Risk Index in Previously Untreated Patients With Advanced NSCLC Treated With a Combination Therapy of Anti-PD-1/-PD-L1 Antibodies and Platinum-Based Chemotherapy: A Multicenter Retrospective Study. Oncology
Socinski MA, Jotte RM, Cappuzzo F et al (2018) Atezolizumab for First-Line treatment of metastatic nonsquamous NSCLC. N Engl J Med 378(24):2288–2301
Cheng Y, Nie L, Liu Y et al (2019) Comparison of Endostar continuous versus intermittent intravenous infusion in combination with first-line chemotherapy in patients with advanced non-small cell lung cancer. Thorac cancer 10(7):1576–1580
Hansma AH, Broxterman HJ, van der Horst I et al (2005) Recombinant human endostatin administered as a 28-day continuous intravenous infusion, followed by daily subcutaneous injections: a phase I and pharmacokinetic study in patients with advanced cancer. Annals Oncology: Official J Eur Soc Med Oncol 16(10):1695–1701
Lu S, Li L, Luo Y et al (2015) A multicenter, open-label, randomized phase II controlled study of rh-endostatin (Endostar) in combination with chemotherapy in previously untreated extensive-stage small-cell lung cancer. J Thorac Oncology: Official Publication Int Association Study Lung Cancer 10(1):206–211
Abdel-Qadir H, Ethier JL, Lee DS et al (2017) Cardiovascular toxicity of angiogenesis inhibitors in treatment of malignancy: a systematic review and meta-analysis. Cancer Treat Rev 53:120–127
Li K, Shi M, Qin S (2018) Current status and study progress of recombinant human endostatin in Cancer Treatment. Oncol Therapy 6(1):21–43
Prager GW, Braemswig KH, Martel A et al (2014) Baseline carcinoembryonic antigen (CEA) serum levels predict bevacizumab-based treatment response in metastatic colorectal cancer. Cancer Sci 105(8):996–1001
Li B, Li C, Guo M et al (2018) Predictive value of LDH kinetics in bevacizumab treatment and survival of patients with advanced NSCLC. OncoTargets Therapy 11:6287–6294
Svaton M, Blazek J, Krakorova G et al (2021) Prognostic role for CYFRA 21– 1 in patients with Advanced-stage NSCLC treated with Bevacizumab Plus Chemotherapy. Anticancer Res 41(4):2053–2058
Bramswig KH, Poettler M, Unseld M et al (2013) Soluble carcinoembryonic antigen activates endothelial cells and tumor angiogenesis. Cancer Res 73(22):6584–6596
Acknowledgements
Not applicable.
Funding
This work was supported by the National Natural Science Foundation of China (No. 41904017), China Post-doctoral Science Foundation (No. 2021M691903), Shandong Provincial Natural Science Foundation (ZR2021LSW015), the Collaborative Innovation Center for Intelligent Molecules with Multi-effects and Nanomedicine (No. 2019-01), Shandong province, China. The funders did not play a role in design and conduct of study, collection and analysis of data, preparation, review, or approval of the manuscript, and decision of submitting the manuscript for publication.
Author information
Authors and Affiliations
Contributions
Conception and design: Xinyi Liu, Degan Lu, Zihan Guo, Lin Su, Xiang Ji, Min Gao.Collection and assembly of data: Xinyi Liu, Zihan Guo, Anli Zuo, Shuran Yang.Data analysis and interpretation: Xinyi Liu, Degan Lu, Lin Su, Anli Zuo, Yunxiu Jiang.Manuscript writing: All authors.Final approval of manuscript: All authors.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was reviewed and approved by the Institutional Review Committee of the First Affiliated Hospital of Shandong First Medical University. Approved No. of ethic committee: YXLL-KY-2020(073). Written informed consent is obtained from all participants. This Trial was registered on ClinicalTrials.gov, NCT05574998.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Liu, X., Guo, Z., Su, L. et al. The efficacy and safety of continuous intravenous infusion of rh-endostatin combined with platinum-based doublet chemotherapy for advanced non-small-cell lung cancer. Invest New Drugs 42, 309–317 (2024). https://doi.org/10.1007/s10637-024-01439-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-024-01439-x