Abstract
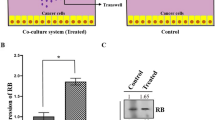
Breast cancer is a leading cause of death in women worldwide. Cancer therapy based on stem cells is considered as a novel and promising platform. In the present study, we explore the therapeutic effects of human amniotic mesenchymal stromal cells (hAMSCs) through the reduction of focal adhesion kinase (FAK) activity, SHP-2, and cell adhesion proteins such as Paxillin, Vinculin, Fibronectin, Talin, and integrin αvβ3 expression in MDA-MB-231 breast cancer cells. For this purpose, we employed a co-culture system using 6-well plate transwell. After 72 h, hAMSCs-treated MDA-MB-231 breast cancer cells, the activity of focal adhesion kinase (FAK) and the expression of SHP-2 and cell adhesion proteins such as Paxillin, Vinculin, Fibronectin, Talin, and integrin αvβ3 expression were analyzed using western blot. The shape and migration of cells were also analyzed. Based on our results, a significant reduction in tumor cell motility through downregulation of the tyrosine phosphorylation level of FAK (at Y397 and Y576/577 sites) and cell adhesion expression in MDA-MB-231 breast cancer cells was demonstrated. Our findings indicate that hAMSCS secretome has therapeutic effects on cancer cell migration through downregulation of FAK activity and expression of cell adhesion proteins.





Similar content being viewed by others
Data availability
No datasets were generated or analysed during the current study.
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68(6):394–424
Zhang CL, Huang T, Wu BL, He WX, Liu D (2017) Stem cells in cancer therapy: opportunities and challenges. Oncotarget 8(43):75756–75766
Sarukhan A, Zanotti L, Viola A (2015) Mesenchymal stem cells: myths and reality. Swiss Med Wkly 145:w14229
Tran C, Damaser MS (2015) Stem cells as drug delivery methods: application of stem cell secretome for regeneration. AdvDrug Deliv Rev 82–83:1–11
Kilroy GE, Foster SJ, Wu X, Ruiz J, Sherwood S, Heifetz A, Ludlow JW, Stricker DM, Potiny S, Green P, Halvorsen YD, Cheatham B, Storms RW, Gimble JM (2007) Cytokine profile of human adipose-derived stem cells: expression of angiogenic, hematopoietic, and proinflammatory factors. J Cell Physiol 212(3):702–709
Caplan AI (2009) Why are MSCs therapeutic? New data: new insight. J Pathol 217:318–324
Sarojini H, Estrada R, Lu H, Dekova S, Lee MJ, Gray RD, Wang E (2008) PEDF from mouse mesenchymal stem cell secretome attracts fibroblasts. J Cell Bioche 104(5):1793–1802
Schinkothe T, Bloch W, Schmidt A (2008) In vitro secreting profile of human mesenchymal stem cells. Stem Cells Dev 17:199–206
Liu CH, Hwang SM (2005) Cytokine interactions in mesenchymal stem cells from cord blood. Cytokine 32:270–279
Ilic D, Furuta Y, Kanazawa S, Takeda N, Sobue K, Nakatsuji N, Nomura S, Fujimoto J, Okada M, Yamamoto T (1995) Reduced cell motility and enhanced focal adhesion contact formation in cells from FAK-deficient mice. Nature 377:539–544
Parsons JT (2003) Focal adhesion kinase: the first ten years. J Cell Sci 116:1409–1416
Schlaepfer DD, Mitra SK, Ilic D (2004) Control of motile and invasive cell phenotypes by focal adhesion kinase. Biochim Biophys Acta 1692:77–102
Calalb MB, Polte RT, Hanks SK (1995) Tyrosine phosphorylation of focal adhesion kinase at sites in the catalytic domain regulates kinase activity: a role for Src family kinases. Mol Cell Biol 15:954–963
Tremblay L, Hauck W, Aprikian AG, Begin LR, Chapde-laine A, Chevalier S (1996) Focal adhesion kinase (pp125) expression, activation and association with paxillin and p50 in human metastatic prostate carcinoma. Int J Cancer 68(2):164–171
Judson PL, He X, Cance WG, Van Le L (1999) Overexpression of focal adhesion kinase, a protein tyrosine kinase, in ovarian carcinoma. Cancer 86(8):1551–1556
Lark AL, Livasy CA, Calvo B, Caskey L, Moore DT, Yang X, Cance WG (2003) Overexpression of focal adhesion kinase in primary colorectal carcinomas and colorectal liver metastases: immunohistochemistry and real-time PCR analyses. Clin Cancer Res 9:215–222
Owens LV, Xu L, Cravenetal RJ (1995) Overexpression of the focal adhesion kinase (p125FAK) in invasive human tumors. Cancer Res 55(13):2752–2755
Safari F, Murata-Kamiya N, Saito Y, Hatakeyama M (2011) Mammalian pragmin regulates Src family kinases via the glu-pro-ile-tyr-ala (EPIYA) motif that is exploited by bacterial effectors. Proc Natl Acad Sci USA 108:14938–14943
Tactacan CM, Phua YW, Liu L, Zhang L, Humphrey ES, Cowley M, Pinese M, Biankin AV, Daly RJ (2015) The pseudokinase SgK223 promotes invasion of pancreatic ductal epithelial cells through JAK1/Stat3 signaling. Mol Cancer 14:139
Senda Y, Murata-Kamiya N, Hatakeyama M (2016) C-terminal src kinase-mediated EPIYA phosphorylation of Pragmin creates a feed-forward C-terminal src kinase activation loop that promotes cell motility. Cancer Sci 107(7):972–980. https://doi.org/10.1111/cas.12962
Rahmani Z, Safari F (2020) Evaluating the in vitro therapeutic effects of human amniotic mesenchymal stromal cells on MiaPaca2 pancreatic cancer cells using 2D and 3D cell culture model. Tissue Cell 23:101479
Tsutsumi R, Takahashi A, Azuma T, Higashi H, Hatakeyama M (2006) Focal adhesion kinase is a substrate and downstream Effector of SHP-2 complexed with Helicobacter pylori CagA. Mol Cell Biol 26(1):261–276
Sieg DJ, Hauck CR, Ilic D, Klingbeil CK, Schaefer E, Damsky CH, Schlaepfer DD (2000) FAK integrates growth-factor and integrin signals to promote cell migration. Nat Cell Biol 2:249–256
Safari F, Shafiee Nejad N, Aghaei Nejad A (2022) The inhibition of Panc1 cancer cells invasion by hAMSCs secretome through suppression of tyrosine phosphorylation of SGK223 (at Y411 site), c-Src (at Y416, Y530 sites), AKT activity, and JAK1/Stat3 signaling. Med Oncol 39:28
Shakery T, Safari F (2023) Downregulation of Pinkbar/pAKT and MMP2/MMP9 expression in MDA-MB-231 breast Cancer cells as potential targets in Cancer Therapy by hAMSCs Secretome. Cells Tissues Organs 212(2):155–163
Biscardi JS, Belsches AP, Parsons SJ (1998) Characterization of human epidermal growth factor receptor and c-Src in human breast tumor cells. Mol Carcinogen 21:261–272
Biscardi JS, Ishizawar RC, Silva CM, Parsons SJ (2000) Tyrosine kinase signalling in breast cancer: epidermal growth factor receptor and c-Src interactions in breast cancer. Breast Cancer Res 2:203–210
Ottenhoff-Kalff AE, Rijksen G, van Beurden EA, Hennipman A, Michels AA, Staal GE (1992) Characterization of protein tyrosine kinases from human breast cancer: involvement of the c-Src oncogene product. Cancer Res 52:4773–4778
Verbeek BS, Vroom TM, Adriaansen-Slot SS, Ottenhoff-Kalff AE, Geertzema JG, Hennipman A, Rijksen G (1996) c-Src protein expression is increased in human breast cancer. An immunohisto-chemical and biochemical analysis. J Pathol 180:383–388
Toi M, Osaki A, Yamada H, Toge T (1991) Epidermal growth factor receptor expression as a prognostic indicator in breast cancer. Eur J Cancer 27:977–980
Thomas SM, Brugge JS (1997) Cellular functions regulated by Src family kinases. Annu Rev Cell Dev Biol 13:513–609
Chen DL, Wang DS, Wu WJ, Zeng ZL, Luo HY, Qiu MZ, Ren C, Zhang DS, Wang ZQ, Wang FH, Li YH, Kang TB, Xu RH (2013) Overexpression of paxillin induced by miR-137 suppression promotes tumor progression and metastasis in colorectal cancer. Carcinogenesis 34:803–811
Chen DL, Wang ZQ, Ren C, Zeng ZL, Wang DS, Luo HY, Wang F, Qiu MZ, Bai L, Zhang DS, Wang FH, Li YH, Xu RH (2013) Abnormal expression of paxillin correlates with tumor progression and poor survival in patients with gastric cancer. J Translational Medici ne 11:277
Hegele A, Heidenreich A, Kropf J, von Knobloch R, Varga Z, Hofmann R, Olbert P (2004) Plasma levels of cellular fibronectin in patients with localized and metastatic renal cell carcinoma. Tumor Biol 25:111–116
Saito N, Nishimura H, Kameoka S (2008) Clinical significance of fibronectin expression in colorectal cancer. Mol Med Rep 1:77–81
Wang Y, Kuramitsu Y, Ueno T, Suzuki N, Yoshino S, Iizuka N, Zhang X, Akada J, Oka M, Nakamura K (2012) Proteomic differential display identifies upregulated vinculin as a possible biomarker of pancreatic cancer. Oncol Rep 28:1845–1850
Kawakami K, Fujita Y, Kato T, Mizutani K, Kameyama K, Tsumoto H, Miura Y, Deguchi T, Ito M (2015) Integrin beta4 and vinculin contained in exosomes are potential markers for progression of prostate cancer associated with taxane - resis-tance. Int J Oncol 47:384–390
Beer AJ, Niemeyer M, Carlsen J, Sarbia M, Nahrig J, Watzlowik P, Wester HJ, Harbeck N, Schwaiger M (2008) Patterns of avb3 expression in primary and metastatic human breast Cancer as shown by 18F-Galacto-RGD PET. J Nucl Med 49(2):255–259
Rolli M, Fransvea E, Pilch J, Saven A, Felding-Habermann B (2003) Activated integrin alpha v beta 3 cooperates with metalloproteinase MMP-9 in regulating migration of metastatic breast cancer cells. Proc Natl Acad Sci USA 100:9482–9487
Liapis H, Flath A, Kitazawa S (1996) Integrin alpha v beta 3 expression by bone-residing breast cancer metastases. Diagn Mol Pathol 5:127–135
Desiniotis A, Kyprianou N (2011) Significance of talin in cancer progression and metastasis. Int R ev Cell Mol Biol 289:117–147
Sakamoto S, McCann RO, Dhir R, Kyprianou N (2010) Talin1 promotes tumor invasion and metastasis via focal adhesion signaling and anoikis resistance. Cancer Res 70:1885–1895
Zhang W, Mao YQ, Wang H, Yin WJ, Zhu SX, Wang WC (2015) MiR-124 suppresses cell motility and adhesion by targeting talin 1 in prostate cancer cells. Cancer Cell Int 15:49
Funding
Not applicable
Author information
Authors and Affiliations
Contributions
F.S. designed the research, performed the experiments, and wrote the paper. F.S., S.B., and F.O.C. analyzed data.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Safari, F., Bararpour, S. & Omidi Chomachaei, F. The suppression of cell motility through the reduction of FAK activity and expression of cell adhesion proteins by hAMSCs secretome in MDA-MB-231 breast cancer cells. Invest New Drugs 42, 272–280 (2024). https://doi.org/10.1007/s10637-024-01434-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-024-01434-2