Abstract
This study examined the activity and safety of amrubicin monotherapy among relapsed small-cell lung cancer (SCLC) patients who had previously been treated with atezolizumab plus carboplatin and etoposide (AteCE). This retrospective study evaluated patients with relapsed SCLC who were treated with previously AteCE combination therapy followed by amrubicin monotherapy between August 2019 and May 2021. Clinical efficacy and toxicity were analyzed. Overall, 40 patients were included: 12 and 28 patients had sensitive and refractory relapse, respectively. The response rate was 32.5% (25.0% in the sensitive group and 35.7% in the refractory group). The median progression-free survival (PFS) and overall survival (OS) from the first amrubicin treatment was 3.4 months (95% CI: 1.9–4.9 months) and 9.9 months (95% CI: 4.5–11.5 months), respectively. There was no significant between-group difference in median PFS (3.6 months vs. 3.2 months, p = 0.42) or median OS (11.2 months vs. 7.3 months, p = 0.78). Grade ≥ 3 hematological adverse events occurred as follows: decreased white blood cells in 52.5% of patients; decreased neutrophil count in 57.5%; and febrile neutropenia in 10.0%. Grade 3 pneumonitis was observed in one patient. There were no treatment-related deaths. Amrubicin is feasible and effective for relapsed SCLC patients previously treated with AteCE therapy. Although immune checkpoint inhibitor treatment (ICI) does not improve the effect of amrubicin, the toxicity is not increased, suggesting that amrubicin remains effective even after ICI administration. Thus, amrubicin after AteCE could be the preferred standard chemotherapeutic choice in patients with relapsed SCLC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lung cancer reportedly accounts for the largest number of cancer deaths globally [1]. Small-cell lung cancer (SCLC) comprises approximately 15% of all lung cancer cases and is an aggressive tumor characterized by prompt doubling time, high proliferation fraction, and early development of widespread metastases [2, 3]. Approximately two-thirds of SCLC cases have extensive disease (ED) at diagnosis, which correlates with poor prognosis [4]. Although most SCLC patients respond to initial treatment, long-term survival is poor. Unfortunately, disease progression or relapse is common [5,6,7,8,9]. Until recently, the standard first-line treatment for patients with ED-SCLC was combination chemotherapy of platinum and etoposide. However, the median overall survival (OS) is limited to approximately 10 months, and OS improvement has not improved in more than 20 years [10, 11]. Before the introduction of immune checkpoint inhibitors (ICIs), the reported outcomes of first-line chemotherapy in patients with ED-SCLC included a median progression-free survival (PFS) of 4.3–5.7 months, a median OS of 7.5–10.9 months, and an average 5-year survival rate of only 2.8% [11, 12].
Recently, ICIs have brought about survival benefits in patients with ED-SCLC [13,14,15,16]. Atezolizumab, which is a humanized monoclonal anti–programmed death ligand 1 (PD-L1) antibody, inhibits PD-L1–programmed death 1 (PD-1) and PD-L1–B7-1 signaling and restores tumor-specific T-cell immunity [17]. The landmark IMpower133 trial showed significantly better survival outcomes in atezolizumab plus carboplatin and etoposide (AteCE) therapy than in carboplatin and etoposide therapy [13, 14]. Moreover, durvalumab, another PD-L1 antibody, showed similar survival efficacy in the CASPIAN trial [15, 16].
Amrubicin and its active metabolite, amrubicinol, are inhibitors of DNA topoisomerase II and exert cytotoxicity by stabilizing cleavable complexes via topoisomerase II rather than via DNA intercalation. Amrubicinol is 5–100 times more active than amrubicin [18]. A previous phase III trial evaluating the activity of amrubicin in relapsed SCLC demonstrated that the overall response rate, PFS, and OS were 31.1%, 4.1 months, and 7.5 months, respectively [19]. Moreover, the response rate, PFS, and OS were 40.9%, 5.5 months, and 9.2 months, respectively, for sensitive cases, and 20.1%, 2.8 months, and 6.2 months, respectively, for refractory cases [19]. The most common adverse event (AE) associated with amrubicin administration is myelosuppression, such as leukopenia and neutropenia, with non-hematologic toxicities occurring less frequently [19,20,21,22,23,24,25,26].
Amrubicin monotherapy is an established standard second-line chemotherapeutic regimen in patients with refractory SCLC [26]. A meta-analysis reported that amrubicin monotherapy is effective in both sensitive and refractory cases of relapsed SCLC [27]. In patients with non-SCLC, overall response rates to cytotoxic single-agent chemotherapy after failure of anti-PD1 therapy are higher than responses to single-agent chemotherapy without prior anti-PD1 therapy [28]. There are also reports of improved treatment response of docetaxel plus ramucirumab therapy beyond nivolumab administration in pretreated non-SCLC [29], supporting the promising efficacy of cytotoxic anticancer drugs after ICIs.
Although systemic treatment consisting of ICIs plus combination chemotherapy with platinum and etoposide is the preferred therapeutic treatment for ED-SCLC, the effectiveness and toxicity of amrubicin in relapsed SCLC patients previously treated with AteCE have not been examined. Thus, the current study aimed to examine the activity and feasibility of amrubicin monotherapy among relapsed SCLC patients who have been pretreated with AteCE.
Patients and methods
Study design and patients
This retrospective study evaluated patients with relapsed SCLC who previously received AteCE combination treatment followed by amrubicin monotherapy between August 2019 and May 2021 in one of nine Japanese institutions. The eligibility criteria were: unresectable clinical stage (III or IV) disease or postoperative recurrence at first-line therapy, cytologically or histologically diagnosed SCLC, and first-line treatment with AteCE combination therapy followed by second-line amrubicin monotherapy.
All patients underwent systematic evaluation and standardized staging procedures before treatment. Clinical stage was assigned based on the results of physical examination, chest radiography, computed tomography (CT) scans of the chest and abdomen, CT or magnetic resonance imaging (MRI) of the brain, and bone scintigraphy or 18F-fluorodeoxyglucose positron emission tomography to assess the TNM stage. Pathological stage III/IV SCLC was determined based on the Union for International Cancer Control tumor-node-metastasis (TNM) Classification, 8th Edition. Sensitive relapse was defined as response to initial anticancer agent treatment and relapse within > 90 days beyond cytotoxic drug treatment. Meanwhile, refractory relapse was defined as no response to initial cytotoxic drug treatment or relapse within 90 days.
This study was approved by the Institutional Review Board of Saitama Medical University International Medical Center (No. 2021–113). All procedures complied with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its subsequent amendments, or comparable ethical standards. Because this is a retrospective study, informed consent was waived.
Treatment and response evaluation
All patients had no history of amrubicin monotherapy, and amrubicin was administered intravenously at dose of 25–45 mg/m2 on days 1 to 3 every 22 or 29 days. Granulocyte colony-stimulating factor was administered as prophylaxis for neutropenia at the discretion of the attending physician, but administration was not mandatory. Amrubicin monotherapy was continued until disease progression, occurrence of unacceptable toxicities, or patient refusal. Radiological tumor responses were assessed based on best overall treatment response and maximum tumor shrinkage according to the Response Evaluation Criteria in Solid Tumors, version 1.1 [30]. Patients who failed treatment were administered subsequent therapy if they wished, including continuation of amrubicin monotherapy. Treatment toxicities related to amrubicin administration were graded following the Common Terminology Criteria for Adverse Events (CTCAE version 5.0).
Statistical analysis
Statistical analyses were performed using the Fisher’s exact test and the Welch’s t-test for categorical and continuous variables, respectively. PFS was calculated from the first day of treatment until progressive disease or any-cause death. OS was calculated from the first day of treatment until death or was censored on the date of the last follow-up. The Kaplan–Meier method was used to estimate survival as a function of time, and survival differences were analyzed using log-rank tests. For the univariable and multivariable prognostic assessments of several clinical important parameters, the Cox proportional hazards regression model was used to calculate hazard ratios (HR) and 95% confidence intervals (CI). For evaluation of correlation, we used the Spearman rank correlation analysis and linear regression analysis. Differences were considered significant at a two-tailed p-value of < 0.05. All statistical analyses were performed using JMP, version 11.0, for Windows (SAS Institute, Cary, NC, USA).
Results
Patient characteristics
Forty patients were treated with AteCE combination chemotherapy followed by amrubicin as second-line chemotherapy. Table 1 shows the patient characteristics. Twelve patients with sensitive relapse and 28 patients with refractory relapse were evaluable for treatment response, survival, and safety. The patients were predominantly male (32, 80.0%), and the median age at the initiation of amrubicin administration was 71 years (range, 57–84 years). Performance status (PS) at the time of amrubicin initiation was 0–1 in 35 patients (87.5%). The median number of prior AteCE cycles was 4 (range, 4–5) in the sensitive group and 4 (range, 2–6) in the refractory group. No patient in the sensitive group and six patients in the refractory group received additional atezolizumab during the course of carboplatin and etoposide combination chemotherapy.
Treatment response and delivery
Treatment response and therapeutic delivery according to the patient group are shown in Table 2. The overall response and disease control rates in the entire cohort were 32.5% (95% CI: 20.0–48.0) and 60.0% (95% CI: 44.5–73.6), respectively. The response rate was 25.0% (95% CI: 8.2–53.8) in the sensitive group and 35.7% (95% CI: 20.6–54.2) in the refractory group. These differences were not significant.
The median number of administration cycles was 3 (range, 1–14) in the sensitive group and 3.5 (range, 1–13) in the refractory group. The most common starting dose was 35 mg/m2/day for both groups. Dose reduction of amrubicin was more frequent at a starting dose of ≥ 40 mg/m2/day than at ≤ 35 mg/m2/day (33.3% [3/9] vs. 12.0% [3/25]).
Survival
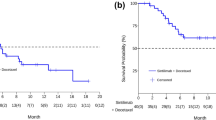
The median follow-up period in the overall population was 6.8 months (range, 1.1–15.9 months), and the median PFS and OS from the initial amrubicin monotherapy for all 40 patients was 3.4 (95% CI: 1.9–4.9) and 9.9 months (95% CI: 4.5–11.5), respectively (Fig. 1a, b). PFS and OS based on relapse pattern is demonstrated in Fig. 2a and b, respectively. The median PFS was 3.6 months in the sensitive group and 3.2 months in the refractory group (p = 0.42). The median OS was 11.2 months in the sensitive group and 7.3 months in the refractory group (p = 0.78). In the univariate analysis, PS was an influencing factor of PFS, and the multivariate analysis showed that PS at the time of amrubicin administration was an independent prognostic factor for PFS. Meanwhile, the number of cycles of atezolizumab maintenance therapy was a prognostic factor for PFS only in the univariate analysis. For OS, univariate and multivariate analyses demonstrated that a PS of 0–1 or ≥ 2 at the initiation of amrubicin administration was an independent prognostic factor for OS (Table 3).
Figure 3 shows the PFS of AteCE, PFS of amrubicin monotherapy, and post-progression survival (PPS) of amrubicin monotherapy in the entire cohort. The relationship of PFS of amrubicin monotherapy with PFS of AteCE therapy and with PPS of amrubicin monotherapy is demonstrated in Fig. 4a and b. Spearman’s rank correlation coefficient and linear regression revealed that the PFS of AteCE therapy was weakly associated with that of amrubicin monotherapy (r = 0.38, p = 0.01, R2 = 0.10), whereas the PPS of amrubicin monotherapy was not associated with that of amrubicin monotherapy (r = 0.06, p = 0.68, R2 = 0.003).
The median PFS of AteCE was 5.1 months (95% CI: 4.4–5.5) for the entire cohort (Online Resource 1). The median OS from the first AteCE combination chemotherapy for the entire cohort was 15.4 months (95% CI: 11.3–18.4) (Online Resource 2).
Toxicity
Treatment-related AEs are shown in Table 4. All 40 patients were assessed for drug-related AEs. The most common treatment-related AE was myelosuppression with 52.5% of patients developing a grade 3–4 decrease in white blood cells, and 57.5% of patients developing a grade 3–4 decrease in neutrophil count. Febrile neutropenia occurred in four patients (10.0%). Grade 3–4 anemia occurred in one patient (2.5%), and a grade 3–4 decrease in platelet count was observed in five patients (12.5%). The rate of non-hematologic AEs was low. The occurrence of immune-related AEs was also low. The most common grade 3–4 non-hematologic AE was infection (7.5%). Grade 3 pneumonitis occurred in one patient. No cardiotoxicity and treatment-related death was observed.
Next, we analyzed hematologic AEs according to administration dose. The grade 3–4 hematologic AEs are shown in Table 4. Compared with patients receiving ≥ 40 mg/m2/day, patients receiving ≤ 35 mg/m2/day showed lower frequencies of decreased neutrophil count (46.4% vs. 66.6%) and decreased white blood cell count (46.4% vs. 66.6%), and higher frequencies of anemia (3.5% vs. 0%) and decreased platelet count (14.2% vs. 8.3%). Febrile neutropenia developed in 10.7% and 8.3% of patients receiving ≤ 35 mg/m2/day and ≥ 40 mg/m2/day, respectively. Hematologic AEs developing at a dose of ≤ 35 mg/m2/day were not necessarily less frequent than those at ≥ 40 mg/m2/day.
Subsequent treatments
Treatments administered following amrubicin monotherapy are shown in Online Resource 3. Among the 35 patients who developed disease progression, 15 patients received anticancer treatments. For subsequent therapy following progressive disease, the most common third-line treatment was topotecan monotherapy (n = 10; 66.6%) followed by irinotecan monotherapy (n = 3; 20.0%). Twenty patients received best supportive care alone. Five patients remained on amrubicin monotherapy or were off amrubicin monotherapy, but showed no evidence of disease progression.
Discussion
In this study, second-line amrubicin monotherapy after AteCE combination chemotherapy showed favorable effectiveness and no new safety concerns. Therapeutic choices for patients with relapsed SCLC remain limited and unclear. Amrubicin is often the treatment of choice for patients with refractory or relapsed SCLC, but the clinical effectiveness and feasibility of amrubicin in patients with relapsed SCLC treated with AteCE have not been assessed. To our best knowledge, this is the first analysis of the treatment effectiveness and feasibility of amrubicin monotherapy for relapsed SCLC patients treated with AteCE therapy.
Patients with SCLC are commonly resistant to cytotoxic drug chemotherapy beyond the first-line setting, but subsequent treatment options are scarce [31]. This study demonstrated that amrubicin monotherapy after AteCE is effective for relapsed SCLC. Amrubicin and topotecan are anticancer drugs that have shown clinical benefit in the second-line setting [19, 21]. Amrubicin has been evaluated in several studies (Table 5); however, there are no reports of amrubicin monotherapy after ICIs. The efficacy of the second-line treatment usually relies on the responsiveness of the tumor to the first-line chemotherapy; that is, whether the tumor is sensitive or refractory.
In previous studies, the overall response rate was higher in sensitive cases (40.9%–70.6%) than in refractory cases (17%–50%). The PFS for sensitive and refractory cases was 3.2–5.5 months and 1.9–4.0 months, respectively, and the OS was 5.5–12.0 months and 4.8–11.0, respectively [19,20,21,22,23,24,25,26, 32]. In the current study, the response rate was 32.5% for all cases, 25.0% for sensitive cases, and 35.7% for refractory cases. Although the current analysis was a retrospective study, the response rate in sensitive cases was inferior to that in other reports. However, the response rate in refractory cases was similar or better than that in other reports, and the response rate was reasonable.
The low response rate in the sensitive group might be attributed to the rather small number of cases (n = 12), differences in patient characteristics, or other biases. However, the PFS and OS in this analysis was not significantly different between sensitive and refractory cases. As shown in Table 5, the PFS and OS were comparable or better in sensitive and refractory cases than in other prospective and retrospective studies reported to date. These results indicate that amrubicin monotherapy after AteCE can be a helpful chemotherapeutic option for chemotherapy-resistant/refractory patients.
In the multivariate analysis of PFS and OS, PS at the beginning of second-line amrubicin monotherapy was an independent prognostic factor for PFS and OS. Although the number of cycles of atezolizumab maintenance therapy (< 2/ ≥ 2) was also found to be correlated with PFS in the univariate analysis, it was not an independent prognostic factor in the multivariate analysis. These findings indicate that amrubicin administration might be effective for improving PFS and OS in refractory or relapsed SCLC patients with favorable PS. The Eastern Cooperative Oncology Group-Performance Status, a subjective scoring system that assesses the general condition of cancer patients, has been reported to be a strong prognostic predictor, showing independent correlations with PFS and OS [33].
The current analysis confirms that PS is a strong prognostic factor, as reported in previous investigations [33], suggesting that our study population reflects the general patient population. The present analysis did not identify the commonly reported relapse pattern (sensitive/refractory) as an independent prognostic factor for either PFS or OS. However, the median PFS and OS of the sensitive group were longer than those of the refractory group, although the difference was not significant (Fig. 2). This result may be due to the small number of cases in both groups. Moreover, prior to the era of ICIs in SCLC, patients who respond to initial pharmacotherapy and have a long interval between completion of initial therapy and relapse (usually ≥ 60–90 days) are often defined as having “sensitive relapse,” while others are defined as “refractory relapse.” Sensitive relapse patients respond better to pharmacotherapy at relapse and survive longer [34, 35]. However, it may be necessary to re-evaluate the criteria for sensitive or refractory relapse after the use of ICIs.
A randomized phase III trial (GFPC01-13) compared oral topotecan monotherapy with carboplatin and etoposide (platinum re-administration) in patients with sensitive relapse after platinum and etoposide therapy. The primary endpoint of PFS was significantly longer in the carboplatin and etoposide group (median: 4.7 months vs. 2.7 months, HR: 0.57, 90% CI: 0.41–0.73, p = 0.0041) [36]. In the current analysis, it might be possible that platinum re-administration may have been the treatment of choice in sensitive relapse cases in the participating institutions, resulting in a relative paucity of amrubicin monotherapy. In addition, univariate analysis demonstrated that PFS was better in the group with more frequent atezolizumab maintenance therapy (≥ 2).
Although multivariate analysis showed no significant difference in the number of cycles of atezolizumab maintenance treatment, there was a weak correlation between the PFS of AteCE and that of amrubicin monotherapy (Fig. 4a). The results suggest that a longer PFS with AteCE also results in a longer PFS for amrubicin monotherapy. Preclinical data suggests that anthracyclines (e.g., amrubicin) as anticancer agents can induce immunogenic cell death in sensitive human tumor cells [37]. However, there are no clinical data reporting the efficacy of anthracyclines (e.g., amrubicin) after ICIs.
As shown in Online Resource 2, the OS from initiation of AteCE was 15.4 months, which compares favorably with the OS in previous phase III trials [13, 14]. A previous report described that PPS has a greater effect on OS than om PFS in refractory SCLC patients who have received second-line amrubicin monotherapy [38]. As shown in Fig. 3 and Online Resource 1, 15 patients received amrubicin monotherapy followed by anticancer drug therapy and 20 patients received best supportive care alone. Topotecan was the most common treatment administered after third-line treatment, followed by irinotecan. Given that etoposide was used for first-line treatment in all patients, it will be necessary to examine the impact of these cytotoxic drug agents on anticancer drug treatment after AteCE combination therapy.
Results of a single-arm phase II trial of pembrolizumab, an ICI, in combination with amrubicin in patients with relapsed SCLC were recently reported [39]. Patients had not received any prior ICIs, but the response rate was 52.0%, the median PFS was 4.0 months, and the median OS was 10.6 months. Additionally, the common grade ≥ 3 AEs were neutropenia (64%), leukopenia (40%), and febrile neutropenia (16%). This report was focused on a regimen of pembrolizumab combined with amrubicin at the time of relapse, and the results showed it was effective and well-tolerated. In the future, it will be necessary to consider whether the treatment strategy for SCLC should be platinum combination chemotherapy with ICIs followed by cytotoxic anticancer agents or platinum combination chemotherapy followed by ICIs and cytotoxic anticancer agents such as amrubicin.
The adverse event profile of amrubicin monotherapy after AteCE noted in the current investigation indicates the feasibility of this modality, similar to previous phase II and III studies in which myelosuppression was observed as the common AE [19,20,21, 24,25,26]. Furthermore, there were no immune-related AEs which are characteristic of amrubicin toxicity after ICI use. The hematologic toxicities that developed were manageable AEs. Overall, non-hematologic AEs were mild. Furthermore, no new adverse event symptoms were observed with amrubicin monotherapy, even after AteCE combination therapy, and no immune-related AEs occurred. The frequency of grade ≥ 3 white blood cell count decrease and neutrophil count decrease was higher at a dose of ≥ 40 mg/m2/day than at a dose of ≤ 35 mg/m2/day. Moreover, dose reduction was more common at a dose of 40 mg/m2/day (Table 2).
As shown in the univariate and multivariate analyses, the initial dose (25–35 or 40–45 mg/m2/day) was not an independent prognostic factor influencing PFS and OS (Table 3). A previous report suggested that among patients with relapsed SCLC, those treated with amrubicin 35 mg/m2 attain comparable clinical benefit, but with less toxicity, than those treated with amrubicin 40 mg/m2 [32]. Therefore, in patients with relapsed SCLC, treatment with amrubicin ≤ 35 mg/m2/day may have comparable effects with less toxicity than treatment with ≥ 40 mg/m2/day or more. Therefore, the optimal dose of amrubicin monotherapy after AteCE combination therapy cannot be determined at this time, but it may not necessarily be > 40 mg/m2/day. This is an issue for further investigation. Pneumonitis was observed in one patient, but this patient recovered with steroid administration. No treatment-related death occurred. These findings indicate that with respect to toxicity, amrubicin monotherapy after AteCE is feasible for relapsed SCLC.
This study had some limitations. First, this was a retrospective study with a small sample size, and larger prospective studies are needed to validate our findings. Second, treatment with anticancer agents was reduced, skipped, or delayed at the discretion of the treating physician. However, we included all consecutive patients treated at the study sites to reduce this bias to the greatest extent possible, and the clinical charts were thoroughly reviewed. Third, the use of AteCE combination chemotherapy for first-line chemotherapy and amrubicin monotherapy for second-line chemotherapy was decided by the treating physician. These decisions could have introduced selection bias, which is an inherent limitation of retrospective studies. The possibility that this may have affected survival could not be ruled out.
In conclusion, amrubicin might be an effective and feasible treatment choice for patients with relapsed SCLC treated with AteCE therapy. Although ICI administration does not improve the effect of amrubicin, it did not enhance toxicity. This indicates that amrubicin is still effective in patients with relapsed SCLC, even after ICI administration. These findings may provide a new direction in the drug treatment of patients with refractory or relapsed SCLC.
Data availability
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
References
Siegel RL, Miller KD, Fuchs HE (2021) Jemal A (2021) Cancer Statistics. CA Cancer J Clin 71(1):7–33. https://doi.org/10.3322/caac.21654
Govindan R, Page N, Morgensztern D, Read W, Tierney R, Vlahiotis A, Spitznagel EL, Piccirillo J (2006) Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: analysis of the surveillance, epidemiologic, and end results database. J Clin Oncol 24(28):4539–4544. https://doi.org/10.1200/JCO.2005.04.4859
Domine M, Moran T, Isla D, Marti JL, Sullivan I, Provencio M, Olmedo ME, Ponce S, Blasco A, Cobo M (2020) SEOM clinical guidelines for the treatment of small-cell lung cancer (SCLC) (2019). Clin Transl Oncol 22(2):245–255. https://doi.org/10.1007/s12094-020-02295-w
Bernhardt EB, Jalal SI (2016) Small Cell Lung Cancer. Cancer Treat Res 170:301–322. https://doi.org/10.1007/978-3-319-40389-2_14
Noda K, Nishiwaki Y, Kawahara M, Negoro S, Sugiura T, Yokoyama A, Fukuoka M, Mori K, Watanabe K, Tamura T, Yamamoto S, Saijo N, Japan Clinical Oncology Group (2002) Irinotecan plus cisplatin compared with etoposide plus cisplatin for extensive small-cell lung cancer. N Engl J Med 346(2):85–91. https://doi.org/10.1056/NEJMoa003034
Hanna N, Bunn PA Jr, Langer C, Einhorn L, Guthrie T Jr, Beck T, Ansari R, Ellis P, Byrne M, Morrison M, Hariharan S, Wang B, Sandler A (2006) Randomized phase III trial comparing irinotecan/cisplatin with etoposide/cisplatin in patients with previously untreated extensive-stage disease small-cell lung cancer. J Clin Oncol 24(13):2038–2043. https://doi.org/10.1200/JCO.2005.04.8595
Lara PN Jr, Natale R, Crowley J, Lenz HJ, Redman MW, Carleton JE, Jett J, Langer CJ, Kuebler JP, Dakhil SR, Chansky K, Gandara DR (2009) Phase III trial of irinotecan/cisplatin compared with etoposide/cisplatin in extensive-stage small-cell lung cancer: clinical and pharmacogenomic results from SWOG S0124. J Clin Oncol 27(15):2530–2535. https://doi.org/10.1200/JCO.2008.20.1061
Turrisi AT 3rd, Kim K, Blum R, Sause WT, Livingston RB, Komaki R, Wagner H, Aisner S, Johnson DH (1999) Twice-daily compared with once-daily thoracic radiotherapy in limited small-cell lung cancer treated concurrently with cisplatin and etoposide. N Engl J Med 340(4):265–271. https://doi.org/10.1056/NEJM199901283400403
Takada M, Fukuoka M, Kawahara M, Sugiura T, Yokoyama A, Yokota S, Nishiwaki Y, Watanabe K, Noda K, Tamura T, Fukuda H, Saijo N (2002) Phase III study of concurrent versus sequential thoracic radiotherapy in combination with cisplatin and etoposide for limited-stage small-cell lung cancer: results of the Japan Clinical Oncology Group Study 9104. J Clin Oncol 20(14):3054–3060. https://doi.org/10.1200/JCO.2002.12.071
Byers LA, Rudin CM (2015) Small cell lung cancer: where do we go from here? Cancer 121(5):664–672. https://doi.org/10.1002/cncr.29098
Farago AF, Keane FK (2018) Current standards for clinical management of small cell lung cancer. Transl Lung Cancer Res 7(1):69–79. https://doi.org/10.21037/tlcr.2018.01.16
Schabath MB, Nguyen A, Wilson P, Sommerer KR, Thompson ZJ, Chiappori AA (2014) Temporal trends from 1986 to 2008 in overall survival of small cell lung cancer patients. Lung Cancer 86(1):14–21. https://doi.org/10.1016/j.lungcan.2014.07.014
Horn L, Mansfield AS, Szczesna A, Havel L, Krzakowski M, Hochmair MJ, Huemer F, Losonczy G, Johnson ML, Nishio M, Reck M, Mok T, Lam S, Shames DS, Liu J, Ding B, Lopez-Chavez A, Kabbinavar F, Lin W, Sandler A, Liu SV, Group IMS (2018) First-Line Atezolizumab plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer. N Engl J Med 379(23):2220–2229. https://doi.org/10.1056/NEJMoa1809064
Liu SV, Reck M, Mansfield AS, Mok T, Scherpereel A, Reinmuth N, Garassino MC, De Castro CJ, Califano R, Nishio M, Orlandi F, Alatorre-Alexander J, Leal T, Cheng Y, Lee JS, Lam S, McCleland M, Deng Y, Phan S, Horn L (2021) Updated Overall Survival and PD-L1 Subgroup Analysis of Patients With Extensive-Stage Small-Cell Lung Cancer Treated With Atezolizumab, Carboplatin, and Etoposide (IMpower133). J Clin Oncol 39(6):619–630. https://doi.org/10.1200/JCO.20.01055
Paz-Ares L, Dvorkin M, Chen Y, Reinmuth N, Hotta K, Trukhin D, Statsenko G, Hochmair MJ, Ozguroglu M, Ji JH, Voitko O, Poltoratskiy A, Ponce S, Verderame F, Havel L, Bondarenko I, Kazarnowicz A, Losonczy G, Conev NV, Armstrong J, Byrne N, Shire N, Jiang H, Goldman JW, CASPIAN investigators (2019) Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet 394(10212):1929–1939. https://doi.org/10.1016/S0140-6736(19)32222-6
Goldman JW, Dvorkin M, Chen Y, Reinmuth N, Hotta K, Trukhin D, Statsenko G, Hochmair MJ, Ozguroglu M, Ji JH, Garassino MC, Voitko O, Poltoratskiy A, Ponce S, Verderame F, Havel L, Bondarenko I, Kazarnowicz A, Losonczy G, Conev NV, Armstrong J, Byrne N, Thiyagarajah P, Jiang H, Paz-Ares L, CASPIAN investigators (2021) Durvalumab, with or without tremelimumab, plus platinum-etoposide versus platinum-etoposide alone in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): updated results from a randomised, controlled, open-label, phase 3 trial. Lancet Oncol 22(1):51–65. https://doi.org/10.1016/S1470-2045(20)30539-8
Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, Sosman JA, McDermott DF, Powderly JD, Gettinger SN, Kohrt HE, Horn L, Lawrence DP, Rost S, Leabman M, Xiao Y, Mokatrin A, Koeppen H, Hegde PS, Mellman I, Chen DS, Hodi FS (2014) Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 515(7528):563–567. https://doi.org/10.1038/nature14011
Tani N, Yabuki M, Komuro S, Kanamaru H (2005) Characterization of the enzymes involved in the in vitro metabolism of amrubicin hydrochloride. Xenobiotica 35(12):1121–1133. https://doi.org/10.1080/00498250500342746
von Pawel J, Jotte R, Spigel DR, O’Brien ME, Socinski MA, Mezger J, Steins M, Bosquee L, Bubis J, Nackaerts K, Trigo JM, Clingan P, Schutte W, Lorigan P, Reck M, Domine M, Shepherd FA, Li S, Renschler MF (2014) Randomized phase III trial of amrubicin versus topotecan as second-line treatment for patients with small-cell lung cancer. J Clin Oncol 32(35):4012–4019. https://doi.org/10.1200/JCO.2013.54.5392
Onoda S, Masuda N, Seto T, Eguchi K, Takiguchi Y, Isobe H, Okamoto H, Ogura T, Yokoyama A, Seki N, Asaka-Amano Y, Harada M, Tagawa A, Kunikane H, Yokoba M, Uematsu K, Kuriyama T, Kuroiwa Y, Watanabe K, Thoracic Oncology Research Group S (2006) Phase II trial of amrubicin for treatment of refractory or relapsed small-cell lung cancer: Thoracic Oncology Research Group Study 0301. J Clin Oncol 24(34):5448–5453. https://doi.org/10.1200/JCO.2006.08.4145
Inoue A, Sugawara S, Yamazaki K, Maemondo M, Suzuki T, Gomi K, Takanashi S, Inoue C, Inage M, Yokouchi H, Watanabe H, Tsukamoto T, Saijo Y, Ishimoto O, Hommura F, Nukiwa T (2008) Randomized phase II trial comparing amrubicin with topotecan in patients with previously treated small-cell lung cancer: North Japan Lung Cancer Study Group Trial 0402. J Clin Oncol 26(33):5401–5406. https://doi.org/10.1200/JCO.2008.18.1974
Shimokawa T, Shibuya M, Kitamura K, Hosomi Y, Hibino S, Ota T, Iguchi M, Okamura T, Gemma A (2009) Retrospective analysis of efficacy and safety of amrubicin in refractory and relapsed small-cell lung cancer. Int J Clin Oncol 14(1):63–69. https://doi.org/10.1007/s10147-008-0802-2
Kim YH, Mio T, Masago K, Irisa K, Sakamori Y, Mishima M (2010) Retrospective analysis of Japanese patients with relapse or refractory small-cell lung cancer treated with amrubicin hydrochloride. Oncol Lett 1(3):569–572. https://doi.org/10.3892/ol_00000101
Ettinger DS, Jotte R, Lorigan P, Gupta V, Garbo L, Alemany C, Conkling P, Spigel DR, Dudek AZ, Shah C, Salgia R, McNally R, Renschler MF, Oliver JW (2010) Phase II study of amrubicin as second-line therapy in patients with platinum-refractory small-cell lung cancer. J Clin Oncol 28(15):2598–2603. https://doi.org/10.1200/JCO.2009.26.7682
Jotte R, Conkling P, Reynolds C, Galsky MD, Klein L, Fitzgibbons JF, McNally R, Renschler MF, Oliver JW (2011) Randomized phase II trial of single-agent amrubicin or topotecan as second-line treatment in patients with small-cell lung cancer sensitive to first-line platinum-based chemotherapy. J Clin Oncol 29(3):287–293. https://doi.org/10.1200/JCO.2010.29.8851
Murakami H, Yamamoto N, Shibata T, Takeda K, Ichinose Y, Ohe Y, Yamamoto N, Takeda Y, Kudoh S, Atagi S, Satouchi M, Kiura K, Nogami N, Endo M, Watanabe H, Tamura T (2014) A single-arm confirmatory study of amrubicin therapy in patients with refractory small-cell lung cancer: Japan Clinical Oncology Group Study (JCOG0901). Lung Cancer 84(1):67–72. https://doi.org/10.1016/j.lungcan.2014.01.012
Horita N, Yamamoto M, Sato T, Tsukahara T, Nagakura H, Tashiro K, Shibata Y, Watanabe H, Nagai K, Nakashima K, Ushio R, Ikeda M, Kobayashi N, Shinkai M, Kudo M, Kaneko T (2016) Amrubicin for relapsed small-cell lung cancer: a systematic review and meta-analysis of 803 patients. Sci Rep 6:18999. https://doi.org/10.1038/srep18999
Schvartsman G, Peng SA, Bis G, Lee JJ, Benveniste MFK, Zhang J, Roarty EB, Lacerda L, Swisher S, Heymach JV, Fossella FV, William WN (2017) Response rates to single-agent chemotherapy after exposure to immune checkpoint inhibitors in advanced non-small cell lung cancer. Lung Cancer 112:90–95. https://doi.org/10.1016/j.lungcan.2017.07.034
Shiono A, Kaira K, Mouri A, Yamaguchi O, Hashimoto K, Uchida T, Miura Y, Nishihara F, Murayama Y, Kobayashi K, Kagamu H (2019) Improved efficacy of ramucirumab plus docetaxel after nivolumab failure in previously treated non-small cell lung cancer patients. Thorac Cancer 10(4):775–781. https://doi.org/10.1111/1759-7714.12998
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45(2):228–247. https://doi.org/10.1016/j.ejca.2008.10.026
Nagy-Mignotte H, Guillem P, Vignoud L, Coudurier M, Vesin A, Bonneterre V, Toffart AC, Sakhri L, Brambilla C, Brambilla E, Timsit JF, Moro-Sibilot D, Multidisciplinary Thoracic Oncology G (2012) Outcomes in recurrent small-cell lung cancer after one to four chemotherapy lines: a retrospective study of 300 patients. Lung Cancer 78(1):112–120. https://doi.org/10.1016/j.lungcan.2012.06.006
Kaira K, Sunaga N, Tomizawa Y, Yanagitani N, Shimizu K, Imai H, Utsugi M, Iwasaki Y, Iijima H, Tsurumaki H, Yoshii A, Fueki N, Hisada T, Ishizuka T, Saito R, Mori M (2010) A phase II study of amrubicin, a synthetic 9-aminoanthracycline, in patients with previously treated lung cancer. Lung Cancer 69(1):99–104. https://doi.org/10.1016/j.lungcan.2009.09.012
Capewell S, Sudlow MF (1990) Performance and prognosis in patients with lung cancer. The Edinburgh Lung Cancer Group. Thorax 45(12):951–956. https://doi.org/10.1136/thx.45.12.951
Ardizzoni A, Hansen H, Dombernowsky P, Gamucci T, Kaplan S, Postmus P, Giaccone G, Schaefer B, Wanders J, Verweij J (1997) Topotecan, a new active drug in the second-line treatment of small-cell lung cancer: a phase II study in patients with refractory and sensitive disease. The European Organization for Research and Treatment of Cancer Early Clinical Studies Group and New Drug Development Office, and the Lung Cancer Cooperative Group. J Clin Oncol 15(5):2090–2096. https://doi.org/10.1200/JCO.1997.15.5.2090
Kim YH, Goto K, Yoh K, Niho S, Ohmatsu H, Kubota K, Saijo N, Nishiwaki Y (2008) Performance status and sensitivity to first-line chemotherapy are significant prognostic factors in patients with recurrent small cell lung cancer receiving second-line chemotherapy. Cancer 113(9):2518–2523. https://doi.org/10.1002/cncr.23871
Baize N, Monnet I, Greillier L, Geier M, Lena H, Janicot H, Vergnenegre A, Crequit J, Lamy R, Auliac JB, Letreut J, Le Caer H, Gervais R, Dansin E, Madroszyk A, Renault PA, Le Garff G, Falchero L, Berard H, Schott R, Saulnier P, Chouaid C, Groupe Français de Pneumo-Cancérologie 01–13 investigators (2020) Carboplatin plus etoposide versus topotecan as second-line treatment for patients with sensitive relapsed small-cell lung cancer: an open-label, multicentre, randomised, phase 3 trial. Lancet Oncol 21(9):1224–1233. https://doi.org/10.1016/S1470-2045(20)30461-7
Fucikova J, Kralikova P, Fialova A, Brtnicky T, Rob L, Bartunkova J, Spisek R (2011) Human tumor cells killed by anthracyclines induce a tumor-specific immune response. Cancer Res 71(14):4821–4833. https://doi.org/10.1158/0008-5472.CAN-11-0950
Imai H, Kaira K, Mori K, Watase N, Hisada T, Yamada M, Minato K (2020) Post-progression survival is strongly linked to overall survival in refractory small-cell lung cancer patients who received amrubicin. J Cancer Res Ther 16(4):764–770. https://doi.org/10.4103/jcrt.JCRT_1170_16
Akamatsu H, Teraoka S, Hayashi H, Fujimoto D, Hayata A, Haratani K, Ozawa Y, Yoshida T, Iwasa T, Shimokawa T, Tomii K, Nakagawa K, Yamamoto N (2021) Pembrolizumab Plus Amrubicin in Patients With Relapsed SCLC: Multi-Institutional, Single-Arm Phase 2 Study. JTO Clin Res Rep 2(7):100184. https://doi.org/10.1016/j.jtocrr.2021.100184
Acknowledgements
The authors thank Ms. Kyoko Nakagawa, Drs. Yukihiro Umeda, Takashi Kasai, Takayuki Kaburagi, Tamotsu Ishizuka, and Kunihiko Kobayashi for their assistance in preparing the manuscript and Editage (www.editage.jp) for English language editing.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Conceptualization and methodology, Hisao Imai; formal analysis and data curation, Hisao Imai and Kyoichi Kaira; Project administration, visualization, and writing–original draft preparation, Hisao Imai; Supervision, Kyoichi Kaira and Hiroshi Kagamu; Investigation and resources, Ayako Shiono, Satoshi Wasamoto, Jun Shiihara, Takeshi Tsuda, Yoshiaki Nagai, Hiroyuki Minemura, Yutaka Yamada, Takayuki Kishikawa, Hirokazu Taniguchi, Ou Yamaguchi, Atsuto Mouri, Kenya Kanazawa, and Koichi Minato; writing –review and editing, all authors. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of Saitama Medical University International Medical Center (No. 2021–113).
Consent to participate
Informed consent is not required because this is a retrospective study.
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Imai, H., Nagai, Y., Minemura, H. et al. Efficacy and safety of amrubicin monotherapy after atezolizumab plus carboplatin and etoposide in patients with relapsed small-cell lung cancer. Invest New Drugs 40, 1066–1079 (2022). https://doi.org/10.1007/s10637-022-01269-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-022-01269-9