Abstract
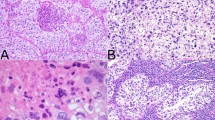
In resected non-small cell lung cancer (NSCLC), ALK rearrangements are associated with worse recurrence-free survival (RFS) than other driver genes. In addition, the micropapillary pattern of NSCLC is associated with a poor prognosis. In recent years, crizotinib tyrosine kinase inhibitors (TKIs) have been widely used to treat patients with advanced NSCLC with ALK fusion. Patient survival outcomes have become highly promising, reflecting the necessity of exploring the application of ALK-TKIs in resected, early stage NSCLC with ALK rearrangements. A 60-year-old Chinese man was diagnosed with stage IIB lung adenocarcinoma harboring a novel SLC8A1/LINC01913 intergenic region-ALK fusion identified by NGS and validated by immunohistochemical staining (IHC) and fluorescence in situ hybridization (FISH). Crizotinib (250 mg orally once daily) was administered to the patient following surgery. The patient remained relapse-free after four months and seven months. This report provided a valuable treatment plan for early lung adenocarcinoma patients with high risks to prevent a postoperative recurrence.



Similar content being viewed by others
Data Availability
Not applicable.
Code Availability
Not applicable.
Change history
16 August 2022
A Correction to this paper has been published: https://doi.org/10.1007/s10637-022-01292-w
References
Tsao AS, Scagliotti GV, Bunn PA Jr, Carbone DP, Warren GW, Bai C, de Koning HJ, Yousaf-Khan AU, McWilliams A, Tsao MS et al (2015) Scientific Advances in Lung Cancer 2015. J Thorac Oncol 11(5):613–638. https://doi.org/10.1016/j.jtho.2016.03.012
Yoshida T, Oya Y, Tanaka K, Shimizu J, Horio Y, Kuroda H, Sakao Y, Hida T, Yatabe Y (2016) Differential Crizotinib Response Duration Among ALK Fusion Variants in ALK-Positive Non-Small-Cell Lung Cancer. J Clin Oncol 34(28):3383–3389. https://doi.org/10.1200/JCO.2015.65.8732
Chaft JE, Dagogo-Jack I, Santini FC, Eng J, Yeap BY, Izar B, Chin E, Jones DR, Kris MG, Shaw AT et al (2018) Clinical outcomes of patients with resected, early-stage ALK-positive lung cancer. Lung Cancer 122:67–71. https://doi.org/10.1016/j.lungcan.2018.05.020
Zeng J, Cui X, Cheng L, Chen Y, Du X, Sheng L (2020) Micropapillary pattern of stage IIIA-N(2) lung adenocarcinoma is a prognostic factor after adjuvant chemoradiotherapy. Future Oncol 16(36):3075–3084. https://doi.org/10.2217/fon-2020-0597
Cai C, Tang Y, Li Y, Chen Y, Tian P, Wang Y, Gong Y, Peng F, Zhang Y, Yu M et al (2021) Distribution and therapeutic outcomes of intergenic sequence-ALK fusion and coexisting ALK fusions in lung adenocarcinoma patients. Lung Cancer 152:104–108. https://doi.org/10.1016/j.lungcan.2020.12.018
Zhu X, He Y, Wang Y, Lei Y, Su X, Liu Y, Wu S, He Z (2021) Identification of a Novel SLC8A1-ALK Fusion and Non-Canonical Expression Significantly Responding to ALK-TKIs in Lung Adenocarcinoma: A Case Report. OncoTargets and therapy 14:4915–4920. https://doi.org/10.2147/OTT.S319845
Xia P, Zhang L, Li P, Liu E, Li W, Zhang J, Li H, Su X, Jiang G (2021) Molecular characteristics and clinical outcomes of complex ALK rearrangements identified by next-generation sequencing in non-small cell lung cancers. J translational Med 19(1):308. https://doi.org/10.1186/s12967-021-02982-4
Boyd JA, Hubbs JL, Kim DW, Hollis D, Marks LB, Kelsey CR (2010) Timing of local and distant failure in resected lung cancer: implications for reported rates of local failure. J Thorac Oncol 5(2):211–214. https://doi.org/10.1097/JTO.0b013e3181c20080
Zhong WZ, Wang Q, Mao WM, Xu ST, Wu L, Shen Y, Liu YY, Chen C, Cheng Y, Xu L (2018) Gefitinib versus vinorelbine plus cisplatin as adjuvant treatment for stage II-IIIA (N1-N2) EGFR-mutant NSCLC (ADJUVANT/CTONG1104): a randomised, open-label, phase 3 study. Lancet Oncol 19(1):139–148. https://doi.org/10.1016/S1470-2045(17)30729-5
Acknowledgements
We thank Ran Ding, Guanghua Lu, Wanglong Deng, Xueyu Yang, and Chuang Qi from Jiangsu Simcere Diagnostics for their kind assistance.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
All authors have made substantial contributions to the conception of the work. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work. Conception/Design: Peiran Xu, Chunguang Wang. Provision of study material or patients: Peiran Xu, Shu Chen. Collection of data: Peiran Xu, Qianru He, Tingting Sun. Data analysis and interpretation: Qianru He, Tingting Sun. Manuscript writing: Chunguang Wang, Qianru He. Final approval of manuscript: Peiran Xu, Chunguang Wang.
Corresponding author
Ethics declarations
Conflict of interest
The authors declared no conflicts of interest.
Ethics approval
All procedures performed in studies involving human participants comply with institutional and/or National Research Council ethical standards and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
A written informed consent was obtained from the patient’s family for publication of the case details.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: the figure images and its legends were missing on the online published paper.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, C., Chen, S., He, Q. et al. A novel SLC8A1/LINC01913 intergenic region-ALK fusion identified by NGS and validated by IHC and FISH in a stage IIB lung adenocarcinoma patient who remains relapse-free during the treatment of crizotinib: a case report. Invest New Drugs 40, 1350–1353 (2022). https://doi.org/10.1007/s10637-022-01262-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-022-01262-2