Abstract
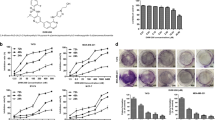
Triple-negative breast cancer (TNBC) is an aggressive subtype of breast cancer that frequently develops resistance to chemotherapy. A new approach to treating TNBC is required to improve patient survival. Phosphodiesterase-4 (PDE4) is an enzyme that is predominantly involved in the modulation of intracellular signaling mediated by cAMP. Although the efficacy of PDE4 inhibitors in several human inflammatory diseases is well documented, their clinical utility has been limited by side effects, including nausea and emesis. Recently, PDE4 has been used as a potential therapeutic target for different cancer types. In the present study, we investigated the anticancer effects of a novel PDE4 inhibitor ZL-n-91 on TNBC and the underlying mechanism. We showed that ZL-n-91 inhibited the proliferation of TNBC cells, induced cell apoptosis, and caused cell cycle arrest. Western blot analysis showed that ZL-n-91 increased Bax level and reduced Bcl-2 expression. Furthermore, downregulation of the cell cycle-related proteins, such as CDK2, CDK4, cyclin D1, PCNA, p-RB, and ZL-n-91, significantly inhibited the transcription of DNA repair genes and triggered an intracellular DNA damage response. Moreover, ZL-n-91 prevented the growth of the transplanted MDA-MB-231 tumor xenograft in nude mice and increased the γ-H2AX expression. These data demonstrate the anticancer effects of ZL-n-91 on TNBC cells and suggest its potential use in anticancer therapy.






Similar content being viewed by others
Data Availability
All of the data generated or analyzed during this study are included in this manuscript.
Code Availability
Not Applicable.
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A et al (2021) Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 71(3):209–249. https://doi.org/10.3322/caac.21660
Harbeck N, Penault-Llorca F, Cortes J, Gnant M, Houssami N, Poortmans P et al (2019) Breast cancer. Nat Rev Dis Primers 5(1):66. https://doi.org/10.1038/s41572-019-0111-2
Nagini S (2017) Breast Cancer: Current Molecular Therapeutic Targets and New Players. Anticancer Agents Med Chem 17(2):152–163. https://doi.org/10.2174/1871520616666160502122724
Vagia E, Mahalingam D, Cristofanilli M (2020) The Landscape of Targeted Therapies in TNBC. Cancers 12(4):916. https://doi.org/10.3390/cancers12040916
Jiang K, Yao G, Hu L, Yan Y, Liu J, Shi J et al (2020) MOB2 suppresses GBM cell migration and invasion via regulation of FAK/Akt and cAMP/PKA signaling. Cell Death Dis 11(4):230. https://doi.org/10.1038/s41419-020-2381-8
Peverelli E, Giardino E, Mangili F, Treppiedi D, Catalano R, Ferrante E et al (2018) cAMP/PKA-induced filamin A (FLNA) phosphorylation inhibits SST2 signal transduction in GH-secreting pituitary tumor cells. Cancer Lett 435:101–109. https://doi.org/10.1016/j.canlet.2018.08.002
Dumaz N, Hayward R, Martin J, Ogilvie L, Hedley D, Curtin JA et al (2006) In melanoma, RAS mutations are accompanied by switching signaling from BRAF to CRAF and disrupted cyclic AMP signaling. Cancer Res 66(19):9483–9491. https://doi.org/10.1158/0008-5472.Can-05-4227
Kim SN, Ahn YH, Kim SG, Park SD, Cho-Chung YS, Hong SH (2001) 8-Cl-cAMP induces cell cycle-specific apoptosis in human cancer cells. Int J Cancer 93(1):33–41. https://doi.org/10.1002/ijc.1308
Sapio L, Gallo M, Illiano M, Chiosi E, Naviglio D, Spina A et al (2017) The Natural cAMP Elevating Compound Forskolin in Cancer Therapy: Is It Time? J Cell Physiol 232(5):922–927. https://doi.org/10.1002/jcp.25650
Bergantin LB (2019) Diabetes and cancer: Debating the link through Ca(2+)/cAMP signalling. Cancer Lett 448:128–131. https://doi.org/10.1016/j.canlet.2019.02.017
Dong H, Claffey KP, Brocke S, Epstein PM (2015) Inhibition of breast cancer cell migration by activation of cAMP signaling. Breast Cancer Res Treat 152(1):17–28. https://doi.org/10.1007/s10549-015-3445-9
Hsien Lai S, Zervoudakis G, Chou J, Gurney ME, Quesnelle KM (2020) PDE4 subtypes in cancer. Oncogene 39(19):3791–3802. https://doi.org/10.1038/s41388-020-1258-8
Wang W, Li Y, Zhu JY, Fang D, Ding HF, Dong Z et al (2016) Triple negative breast cancer development can be selectively suppressed by sustaining an elevated level of cellular cyclic AMP through simultaneously blocking its efflux and decomposition. Oncotarget 7(52):87232–87245. https://doi.org/10.18632/oncotarget.13601
Lin DC, Xu L, Ding LW, Sharma A, Liu LZ, Yang H et al (2013) Genomic and functional characterizations of phosphodiesterase subtype 4D in human cancers. Proc Natl Acad Sci U S A 110(15):6109–6114. https://doi.org/10.1073/pnas.1218206110
Feng X, Wang H, Ye M, Xu XT, Xu Y, Yang W et al (2018) Identification of a PDE4-Specific Pocket for the Design of Selective Inhibitors. Biochemistry 57(30):4518–4525. https://doi.org/10.1021/acs.biochem.8b00336
Zheng S, Kaur G, Wang H, Li M, Macnaughtan M, Yang X et al (2008) Design, synthesis, and structure-activity relationship, molecular modeling, and NMR studies of a series of phenyl alkyl ketones as highly potent and selective phosphodiesterase-4 inhibitors. J Med Chem 51(24):7673–7688. https://doi.org/10.1021/jm701635j
Wang YJ, Jiang YL, Tang HF, Zhao CZ, Chen JQ (2010) Zl-n-91, a selective phosphodiesterase 4 inhibitor, suppresses inflammatory response in a COPD-like rat model. Int Immunopharmacol 10(2):252–258. https://doi.org/10.1016/j.intimp.2009.11.008
Tang HF, Lu JJ, Tang JF, Zheng X, Liang YQ, Wang XF et al (2010) Action of a Novel PDE4 inhibitor ZL-n-91 on lipopolysaccharide-induced acute lung injury. Int Immunopharmacol 10(4):406–411. https://doi.org/10.1016/j.intimp.2010.01.003
Srinivas US, Tan BWQ, Vellayappan BA, Jeyasekharan AD (2019) ROS and the DNA damage response in cancer. Redox Biol 25:101084. https://doi.org/10.1016/j.redox.2018.101084
Dai L, Tian S, Zhang J, Lu M, Zhu J, Zhao H (2021) F1012-2 Induced ROS-Mediated DNA Damage Response through Activation of MAPK Pathway in Triple-Negative Breast Cancer. Biomed Res Int 2021:6650045. https://doi.org/10.1155/2021/6650045
Bianchini G, Balko JM, Mayer IA, Sanders ME, Gianni L (2016) Triple-negative breast cancer: challenges and opportunities of a heterogeneous disease. Nat Rev Clin Oncol 13(11):674–690. https://doi.org/10.1038/nrclinonc.2016.66
Keenan TE, Tolaney SM (2020) Role of Immunotherapy in Triple-Negative Breast Cancer. J Natl Compr Canc Netw 18(4):479–489. https://doi.org/10.6004/jnccn.2020.7554
Ramezani S, Hadjighassem M, Vousooghi N, Parvaresh M, Arbabi F, Amini N et al (2017) The Role of Protein Kinase B Signaling Pathway in Anti-Cancer Effect of Rolipram on Glioblastoma Multiforme: An In Vitro Study. Basic Clin Neurosci 8(4):325–336. https://doi.org/10.18869/nirp.bcn.8.4.325
Ma R, Yang BY, Wu CY (2008) A selective phosphodiesterase 4 (PDE4) inhibitor Zl-n-91 suppresses IL-17 production by human memory Th17 cells. Int Immunopharmacol 8(10):1408–1417. https://doi.org/10.1016/j.intimp.2008.05.012
Nie L, Wei Y, Zhang F, Hsu YH, Chan LC, Xia W et al (2019) CDK2-mediated site-specific phosphorylation of EZH2 drives and maintains triple-negative breast cancer. Nat Commun 10(1):5114. https://doi.org/10.1038/s41467-019-13105-5
Klein MA (2020) Cyclin-dependent kinase inhibition: an opportunity to target protein-protein interactions. Adv Protein Chem Struct Biol 121:115–141. https://doi.org/10.1016/bs.apcsb.2019.11.009
Bertoli C, Skotheim JM, de Bruin RA (2013) Control of cell cycle transcription during G1 and S phases. Nat Rev Mol Cell Biol 14(8):518–528. https://doi.org/10.1038/nrm3629
Johnson J, Thijssen B, McDermott U, Garnett M, Wessels LF, Bernards R (2016) Targeting the RB-E2F pathway in breast cancer. Oncogene 35(37):4829–4835. https://doi.org/10.1038/onc.2016.32
Zhu Y, Ke KB, Xia ZK, Li HJ, Su R, Dong C et al (2021) Discovery of vanoxerine dihydrochloride as a CDK2/4/6 triple-inhibitor for the treatment of human hepatocellular carcinoma. Mol Med 27(1):15. https://doi.org/10.1186/s10020-021-00269-4
Ogawa R, Streiff MB, Bugayenko A, Kato GJ (2002) Inhibition of PDE4 phosphodiesterase activity induces growth suppression, apoptosis, glucocorticoid sensitivity, p53, and p21(WAF1/CIP1) proteins in human acute lymphoblastic leukemia cells. Blood 99(9):3390–3397. https://doi.org/10.1182/blood.v99.9.3390
Chen L, Gao H, Liang J, Qiao J, Duan J, Shi H et al (2018) miR-203a-3p promotes colorectal cancer proliferation and migration by targeting PDE4D. Am J Cancer Res 8(12):2387–2401
Lacraz G, Figeac F, Movassat J, Kassis N, Portha B (2010) Diabetic GK/Par rat beta-cells are spontaneously protected against H2O2-triggered apoptosis. A cAMP-dependent adaptive response. Am J Physiol Endocrinol Metab 298(1):E17–E27. https://doi.org/10.1152/ajpendo.90871.2008
Cho EA, Juhnn YS (2012) The cAMP signaling system inhibits the repair of γ-ray-induced DNA damage by promoting Epac1-mediated proteasomal degradation of XRCC1 protein in human lung cancer cells. Biochem Biophys Res Commun 422(2):256–262. https://doi.org/10.1016/j.bbrc.2012.04.139
Ben-Shlomo A, Deng N, Ding E, Yamamoto M, Mamelak A, Chesnokova V et al (2020) DNA damage and growth hormone hypersecretion in pituitary somatotroph adenomas. J Clin Invest 130(11):5738–5755. https://doi.org/10.1172/jci138540
Acknowledgements
This work was supported by grants from the National Key R&D Program of China (2018YFA0800600); the National Natural Science Foundation of China (81630021); Key Research and Development Program of Guangdong Province for “Innovative drug creation” (2019B020201015); the Guangdong Innovative Research Team Program (2016ZT06Y432); the National Program on Key Basic Research Project of China (2013CB945202); the Startup R&D Funding of Guangdong University of Technology (50010102); and the GuangZhou Basic and Applied Basic Research Foundation (202102021117, 202102020159).
Author information
Authors and Affiliations
Contributions
Z.S.J, Z.Z.G, and L.F.H designed and supervised the study. L.L.M conducted the experiments, analyzed the data, and wrote the manuscript. C.H.S and M.P conducted the experiments and analyzed the data. L.Y.Y, X.L.J, H.Y.J, and M.Y.P assisted with the animal experiments. Z.Z.G and L.F.H conceived and organized the manuscript. Z.S.J and A.Z.Z reviewed and edited the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors have declared that no conflicts of interest exists.
Ethics approval
This study does not include any human experiments.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liang, L., Chen, H., Mao, P. et al. ZL-n-91, a specific Phosphodiesterase-4 inhibitor, suppresses the growth of triple-negative breast cancer. Invest New Drugs 40, 875–883 (2022). https://doi.org/10.1007/s10637-022-01258-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-022-01258-y