Abstract
Glioblastoma multiforme (GBM) is a malignant CNS tumor with a poor prognosis. GBM shows aberrant glycosylation with hypersialylation. This property is a potential target for therapy. This study investigates the growth inhibitory efficacy of poly-guanidine (GuaDex), with an affinity for sialic acid (Sia). Glioma cell cultures and patient-derived glioma cell lines (PDGCLs) expressing Prominin-1 (CD133) were used. Human fibroblasts and astrocyte-derived cells were used as controls. Temozolomide (standard GBM drug, TMZ) and DMSO were used as a comparison. GuaDex at 1–10 µM concentrations, were incubated for 3.5–72 h and with PDGCLs cells for 6–24 h. The cytotoxicity was estimated with a fluorometric cytotoxicity assay (FMCA). Fluorescence-labelled GuaDex was used to study the cell interactions. Sia expression was confirmed with a fluorescence labelled Sia binding lectin. Expression of glial fibrillary acidic protein was determined. GuaDex induction of growth inhibition was fast, showing after less than 5 min incubation while the control cells were not affected even after 50 min incubation. The growth inhibitory effect on PDGCLs spheroids was persistent still showing after 4 weeks post-treatment. The growth inhibition of GuaDex was induced at low µM concentrations while TMZ induced only a slight inhibition at mM concentrations. GuaDex efficacy appears significant and warrants further studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glioblastoma multiforme (GBM) is a highly malignant central nervous system (CNS) tumor and remains incurable. The prognosis is bleak with less than 5% of diagnosed patients alive after 5 years. Median survival time post diagnosis is approximately 15 months. The presumed cellular origin is astrocytes and GBM is the most aggressive of astrocytic tumors. The etiology of glioma remains largely unknown. The vast majority of GBM is located in the supratentorial cerebral hemispheres and only a few percent in cerebellum, brainstem and spinal cord. Imaging techniques are used for diagnosis of gliomas, primarily magnetic resonance scanning (MR), also with gadolinium enhancement. Usual finding is a unifocal lesion with central necrosis surrounded by edema [1].
The principal treatment of GBM is surgery followed by radiation and chemotherapy. However, all treatment is restricted by three principal inherent factors, namely the tumors delicate location i.e., risking damage to healthy brain tissue resulting in devastating side effects, the blood brain barrier (BBB) and last, the properties of the tumor itself i.e., infiltrative growth and a general treatment resistance. Surgery will, importantly, temporarily alleviate symptoms (e.g., mass effects, neurological symptoms, seizures etc.) and like this improve quality of life of the patient. However, the infiltrative growth of GBM, leaving no clear border between tumor and normal brain tissue, precludes radical/curative surgery. Consequently, and despite added chemotherapy and radiation therapy, occurrence happens in most cases in and around the margin of the resected lesion [2]. Likewise, the delicacy of the surrounding healthy brain tissue and the tumors infiltrative growth, limits the effectiveness of radiotherapy to avoid severe side effects (radiation necrosis, nerve cell damage etc.). The BBB carefully regulates entrance to the parenchymal area of the CNS through its special semi permeable capillaries discriminating between molecular weight and chemical properties etc. [3]. Thus, BBB limits which drugs that may be used for systemic therapy. Nitrosourea compounds have been used to treat GBM however with transient and modest efficacy and with significant side effects [4]. Temozolomide (TMZ) is a triazene and is rapidly converted to an active form at physiologic pH to mono-methyl-triazene -imidazole- carboxamide (MTIC, a DNA methylator). TMZ is at the present the standard chemotherapeutic drug for systemic treatment of GBM [5]. The efficacy is modest and with several serious side effects. In addition, GBM appears in itself very resistant to any treatment apparent also when investigating efficacy of drugs in vitro and under ideal conditions. This was obvious also in this study.
Hence, the factors affecting treatment of GBM are unfavourable and new approaches are highly needed to improve the prognosis of this patient category.
Aberrant glycosylation resulting in hyper-sialylation is a general feature of malignant cells that differentiate them from normal cells. This feature may offer a target for specific treatment. Hyper-sialylation is advantageous to the tumor cells facilitating invasion/infiltration, evasion of the immune system and confers resistance to chemo/radiation therapy [6, 7].
One significant consequence of the hyper-sialylation is a strong anionic electrostatic charge, which may serve as a primary, and tumor selective target [8]. Cationic cytotoxic compounds such as poly-guanidine (GuaDex, by definition a polyamine) can be electrostatically attracted to the anionic tumor and be internalized via the polyamine uptake system and when intracellular, induce significant toxicity (9). The polyamine hunger expressed by malignant cells reflects their elevated metabolic needs [10, 11].
This in vitro study investigates the efficacy of GuaDex on GBM cell cultures and the efficacy is compared to the standard chemotherapeutic drug for treatment of GBM.
Materials and methods
Serum based cell culture of glioma cell lines
Glioma cell lines U373MG, U251MG, U1242MG, U343MG and U343MGa Cl2.6, (Table 1) were from the Prof. Monica Nister laboratory, CCK, Karolinska Institutet. The cells were cultured in Iscoves Modified Dulbeccos medium (IMDM) containing 10% fetal bovine serum, antibiotics (100 µg of penicillin and 50 µg of streptomycin sulphate/mL), and 2 mM glutamine at 37 °C, 5% CO.
Glioma patient-derived cell lines (PDGCLs)
BT112 and BT179 PDGCLs expressing Prominin-1 (CD133) (positive marker for stem cells) (Table 1) were a kind gift from Dr. Keith L. Ligon (Dana-Farber Cancer Institute, Harvard Medical School, Boston, MA, USA). Briefly, 500–1000 k PDGCLs cells were growth using Corning® Ultra-Low attachment cell culture flasks 75 cm2 in Stem cell Technologies NeuroCult® NS-A Proliferation Kit (Human) supplanted with 2 µg/mL Heparin, human rh EGF 20 ng/mL, human rh bFGF 20 ng/mL and antibiotics (100 µg penicillin and 50 µg streptomycin sulphate/mL), at 37 °C, 5% CO. The PDGCLs sphere cultures were treated in Corning® Ultra-Low attachment 6 well culture plates (Sigma-Aldrich, St. Louis MO, USA). BT112 and BT179 monolayer cultures were grown in 96-well microtiter plates (Falcon; Becton Dickinson, Mylan, France) coated with laminin (Sigma-Aldrich, St. Louis MO, USA).
Fibroblast and astrocyte cell cultures
Normal human diploid dermal fibroblasts (NHDF-c, lot #1 × 0083002.2) derived from juvenile foreskin were used as negative controls, (Promocell, Heidelberg, Germany). Fibroblast were cultured in Iscoves Modified Dulbecco’s Medium (IMDM with L-glutamine and HEPES) containing 10% fetal bovine serum, antibiotics (100 µg of penicillin and 50 µg of streptomycin sulphate/mL), and 2 mM glutamine at 37 °C, 5% CO. A second negative control was immortalized astrocytes, a gift from Dr. Linda Sleire (Department of Biomedicine, Bergen University, Norway). Astrocytes were cultured in DMEM/high glucose with L-glutamine and sodium pyruvate, non-essential amino acids 1x, 10% fetal bovine serum and antibiotics (100 µg of penicillin and 50 µg of streptomycin sulphate/mL).
Lectin binding studies
A fluorescein-elderberry bark lectin Sambucus nigra (SNA) (ImmunKemi F & D AB, Järfälla, Sweden) was used as SIA expression control ligand. Glioma and fibroblast cells cultures were seeded onto 8-well glass chamber slides (Falcon, Coning, NY, USA) and the PDGCLs 2D cells were seeded onto glass chambers slices covered with laminin all of them at 37 °C for 24 h. PDGCLs spheres were cultured in Ultra-Low attachment 6 well plates. The cells were rinsed with PBS two times. Twenty µg/mL of SNA lectin in PBS was added to all monolayer cells and PDGCLs spheres which were then incubated for 2 h in the dark at room temperature. After the incubation the cells were rinsed with PBS three times. The monolayer cell cultures were fixed for 15 min in 70% ethanol and mounted in mounting medium with DAPI (Vectashield®, ImmunKemi F & D AB, Järfälla, Sweden). The PDGCLs spheres were fixed, frozen and then sectioned as described below. Antigen retrieval was performed by 15 min incubation in 70% ethanol. Slides were then mounted using mounting media containing DAPI. A confocal microscope (Leica SP5, with a Leica Application Suite advance florescence 2011 Software, Leica Microsystems GmbH, Wetzlar, Germany) was used for image analysis.
GuaDex binding studies
GuaDex was prepared as described previously [9], briefly; aminoguanidine was coupled to oxidized dextran followed by reductive amination. The conjugate was purified by gel filtration on a PD-10 disposable Sephadex G-25 column (GE Healthcare, Uppsala, Sweden).
GuaDex is a cationic polydisperse carbohydrate polymer with conjugated guanidine groups. The average molecular weight is 25 kD.
Rhodamine-labelling of GuaDex was made with 500 ug rhodamine B isothiocyanate (Sigma-Aldrich, St. Louis MO, USA) mixed with 1 mL of GuaDex (15 mg/mL), in 0.02 M borate buffer at pH 9.5. FITC-labelling of GuaDex was made with 300 ug FITC (Sigma-Aldrich, St. Louis MO, USA) mixed with 1 mL of GuaDex (15 mg/mL), in 0.02 M borate buffer at pH 9.5. The solutions were incubated on a shaker, overnight in the dark at room temperature. Then the solutions were purified on a PD-10 column equilibrated with PBS. The glioma cells and astrocytes were seeded onto 8-well glass chamber slides at 37 °C for 24 h. PDGCLs spheroids were media without heparin seeded in Ultra-Low attachment 6-well plates. Rhodamine-GuaDex or FITC-GuaDex at 5 µM concentration were added to glioma cells, astrocytes, and sphere cultures. The cells were incubated 4 h in the dark at 37 °C. Then glioma cells and astrocytes were rinsed with PBS three times fixed with 70% ethanol and rinsed 2 times with PBS. Glioma cells and astrocytes were mounted on glass slides using mounting media containing DAPI. PDGCLs spheroids were fixed, frozen, sectioned and mounted on glass slides. A confocal microscope (Leica SP5) was used for the images analysis.
Tissue fixation of PDGCLs spheres
PDGCLs spheroids after lectin and GuaDex binding were re-suspended in 4% paraformaldehyde (PFA) in PBS (pH 7.0) and incubated overnight and then transferred to a 0.5 M sucrose/PBS solution for cryoprotection. The tissue was embedded in Tissue-Tek O.C.T. compound (Sakura® Finetek, Europe B.V.). Frozen sections were cut at 7 µm on a cryostat and harvested on adhesion microscope slides (Superfrost Plus™, Fisher Scientific™, Pittsburgh, PA, USA).
Glial fibrillary acidic protein (GFAP) expression
The GFAP expression was determined to investigate if this protein could affect the GuaDex cell uptake. Glioma cells were seeded on glass cover slips for 24 h, formalin fixed for 10 min and washed in PBS. Cells were permeabilized with 0.1% Triton-X in PBS for 10 min at 4 °C and washed again with PBS. Frozen section antigen retrieval was performed by 20 min incubation in 10 mM citrate buffer at 90 °C after which slides were washed in PBS. Slides and cover slips were incubated with 1:500 GFAP IgG (DAKO, Z0334) in PBS, containing 0.5% BSA for 2 h at room temperature. Then the slides were washed 3 times with PBS and incubated with a secondary antibody. Texas Red goat anti-rabbit (Vectashield, Immunkemi F & D AB, Järfälla, Sweden) 1:500 was added to the sample for 1 h. Finally, the slides were washed 3 times with PBS and mounted in mounting medium containing DAPI. An inverted confocal microscope (Zeiss 710) with ZEN 2009 Software, were used for image analysis and the percentage of positive cell to GFAP expression was manually quantified.
Fluorimetric cytotoxicity assay (FMCA)
The assay was performed as described previously [12]. Briefly, ~ 8,000 glioma or glioma patient-derived cells ~ 10,000 fibroblast or astrocytes/well were seeded onto 96-well microtiter plates (Falcon; Becton Dickinson, Meylan, France) 24 h before treatment. Temozolomide (TMZ) (Sigma-Aldrich, St. Louis MO, USA) drug control was dissolved in DMSO at 0.6 to 1.2 mM concentration. The drug control was tested as well the U343MG Cl2.6 and U1242MG and U251MG glioma cells for 24 h. Equal volume 100% DMSO and PBS were used as controls. GuaDex was tested at 5 µM incubated 1 min to 6 h and at 0.5 µM to 10 µM incubated for 24 -72 h. The control wells were furnished with PBS. All the experiments were done with six-duplicates wells. After incubation the medium was removed by flicking the plates. The cells were washed in PBS. Fluorescein diacetate (FDA) (Sigma-Aldrich, St. Louis MO, USA) was dissolved in DMSO and kept frozen at -20 °C as a stock solution (10 mg/mL). The FDA was diluted in PBS at 10 μg/mL, 200 μl was added to each well. The plates were then incubated for 30 min at 37 °C. A 96-well scanning fluorometer (Infinite® M1000Pro-Tecan, Grödig/Salzburg, Austria) was used to count the emitted fluorescence of all the monolayer cells. The results were calculated using Microsoft Excel. The GuaDex CI50 was defined as the concentration resulting in 50% decrease in cell viability.
GuaDex long-term effect on PDGCLs spheroids
Fluorometric cytotoxicity assay adapted for spheroids (S-FMCA)
Spheroid viability measurements is a challenge because of the variability of spheroid morphology and varying sizes. Semi-quantitative or semi-qualitative assessment of PDGCLs viability have been used to study cytotoxicity of drugs on spheroids, measuring spheroid numbers and spheroid size, by manual counting spheroid dissociated cells after trypan blue viability staining [13], by low- or high-resolution imaging [14], and by homogeneous-sized and shaped spheroids fabrication [15]. These methods are complicated to perform and time-consuming.
A more user-friendly S-FMCA method was constructed for testing the cytotoxicity of GuaDex on spheroids and to investigate the long-term growth inhibitory effect on BT112 and BT1179 PDGCLs spheroid cultures.
The spheroid samples were incubated in 2 mL media with and without heparin (heparin being electronegative potentially could react with GuaDex) in Corning Ultra-Low attachment 6 well plates. Previous treatment, spheroids were split carefully in 2 equal volumes, one for control and the second for treatment test (2 mL each). The test spheroids were treated with 5 µM GuaDex and incubated for 48 h at 37 °C. The control wells were furnished with the media. All the experiments were done in four duplicates. After 48 h the control and test spheroids were centrifuged in tubes at 1000 rpm for 5 min, the supernatant was discarded, washed once in 1 mL PBS and then re-suspended in 2 mL new media. After 2 weeks each were split carefully into 2 equal parts. One part (1 mL) is continued 4 weeks growth and the second part (1 mL) to measure viability. The samples and controls were centrifuged (1000 rpm for 5 min) and washed in PBS as before and the supernatant discarded. A solution of 1 mL FDA (10 μg/mL) solution was added to and then gently vortexed to ensure that the spheroid cells were dissociated in suspension for incubation at 37 °C for 30 min. Then the tubes were gently vortexed again to ensure that all the spheroid cells were in a suspension and dispersed in a cuvette. The S-FMCA was measured at 490 nm using a spectrophotometer (Genesys™ 10S Thermo Scientific™, Waltham, MA, USA). The remaining spheroids were re-plated to grow in 2 mL new media for a total of 4 weeks. Then, each full volume sample (2 mL) was collected and repeated the procedure as after 2 weeks incubation preparing each sample for S-FMCA measurement.
Images of the PDGCLs spheroids incubated with GuaDex were obtained following the experiment of the S-FMCA measurement. An inverted microscope Leica DM IL LED, (Leica microsystems, Wetzlar, Germany) was used to obtain images at 10 min, 24 h, 1- 2- and 4-weeks incubation of GuaDex on BT112 and BT179 PDGCLs spheroids.
Results
Monolayer cells
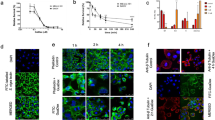
In general, the cellular uptake of GuaDex was enhanced and correlated with the expression of Sia but also to some extent with GFAP expression (fluorescent labelled GuaDex, Fig. 1). Astrocytes and U343MG with low/no expression of these markers showed very low uptake of GuaDex (Fig. 1a).
Confocal microscopy images: a) monolayers glioma cell lines, GFAP expression, Sialic acid α-2,6-Gal expression FITC-labelled S. nigra lectin, Rhodamine-GuaDex binding, monolayer BT112 and BT179 PDGCLs, and fibroblasts, sialic acid expression, DAPI staining, nucleus (blue); b) corresponding at BT112 and BT179 PDGCLs spheroids. Magnification 40x
Spheroids
Similar correlation was observed in spheroids. BT179 with low expression of both markers showed low uptake of GuaDex. Larger spheroids (~ 0.8 mm) showed less uptake compared to smaller spheroids (~ 0.5 mm) showing strong uptake (Fig. 1b).
GuaDex-FITC tumor cell interaction
The Fig. 2 showed the mechanism of the interaction of GuaDex-FITC with U343MG cell membrane and nuclear membrane.
Confocal microscopy, images at 4 h incubation at 5 µM FITC-GuaDex U343MG binding, a) membrane adsorption; b) and c) absorption /internalization, nuclear membrane perforations, nuclear fragmentation, cell membrane collapse and nuclear collapse/DNA precipitation. DAPI staining, nucleus (blue). Magnification 40x
Growth inhibition efficacy
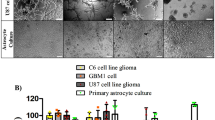
GuaDex showed superior efficacy compared to TMZ where TMZ showed no effect at equimolar concentrations (0.5–10 µM). TMZ required 100 times higher concentration to show efficacy (Fig. 3a). GuaDex efficacy was time and concentration dependent (Fig. 3b, c, d and e). The GuaDex IC50 at treatment on glioma cells lines was 2.2- 7.4 µM at 24 h and 1.2–3.0 µM at 72 h. The IC50 at 24 h was 2.2–2.5 µM on monolayer glioma patient-derived cell lines (PDGCLs).
FMCA cytotoxicity assay, a) GuaDex and temozolomide compared, 24 h incubation at different concentrations. Control, 100% DMSO; b), d) and e) monolayer glioma cells incubated at 5 µM GuaDex during 3.5 h, 24 h, and 72 h.; c) and d) monolayer BT112 and BT179 PDGCLs, astrocytes and fibroblast incubated at 5 µM GuaDex during 6 h and 24 h
Lasting growth inhibition on PDGCLs spheroids
GuaDex was incubated with the spheroids for 48 h, washed and then tested for viable cells after 2 weeks and 4 weeks post treatment. The results showed a prolonged growth inhibition effect, < 7% viable, 2 weeks post treatment and 23–48% viable cells after 4 weeks post treatment. If media contained heparin, there seemed to be certain inhibition of the GuaDex efficacy (heparin is electronegative may interact with GuaDex). There was no regrowth in BT112 while BT179 showed new proliferation (Fig. 4a and b).
a) Spheroid fluorometric cytotoxicity assay (S-FMCA), 2 and 4 weeks, long-term growth inhibitory effect after an initial 48 h incubation at 5 µM GuaDex concentration, with and without heparin containing media, BT112 and BT179 PDGCLs spheroids. b) Inverted microscope images, long-term inhibitory effect after an initial 48 h incubation at 5 µM GuaDex concentration, without heparin containing media on BT112 and BT179 PDGCLs spheroids. Magnification 20x
GuaDex
The schematic molecular formula of GuaDex is illustrated in Fig. 5.
Discussion
During the last decade there has been an upsurge in research on GBM resulting in new insights in molecular mechanisms and genetic mutations/changes. New therapeutic strategies have been suggested and are actively being investigated, such as gene therapy, immunotherapy, and stem cell therapy [16]. There are also several targeted therapies that have been put forward for consideration as novel treatment options, for example immune checkpoint targeting inhibitors [17]. The use of nano technology for the delivery of cytotoxic entities to the GBM target has also been suggested [18]. The use of electric fields applied by an external device, tumor treating fields (TTF), has been developed with some positive results [19].
However, despite considerable improvement of the understanding of the GBM pathology with several connected treatment suggestions and further developments in radiosurgery & radiation technology, GBM resists treatment and prognosis remains poor.
There are obviously a number of circumstances and reasons for this apparent lack of significant progress in GBM treatment. Maybe the most important obstacle is its delicate location together with its infiltrative growth pattern, surrounded with vital brain tissue precluding radical interventions. Further complicating is the BBB that limits the possibilities for systemic therapy although there are claims that BBB is disrupted in GBM allowing systemic therapy [20].
However, there are also several other limiting factors that explain the shortage of new effective GBM therapies. Translational cancer drug research usually means the translation from "the lab bench" to clinical research that eventually may result in a market approved new prescription drug. This translation is a most difficult endeavour with low, or very low success rates. The translation process of novel cancer drug candidates is described as "crossing the valley of death" i.e., death of drug candidates [21]. The US food and drug administration (FDA) and European medicines agency (EMA) usually require animal studies before a cancer drug candidate may enter human clinical research but less than 8% translates in human positive results in clinical trials. Of those projects that enter clinical research 85% fail in the early phases and of those that make it to phase III only 50% are finally approved for clinical use [22]. The same very modest success rates are noted also for immunotherapies (e.g., cancer vaccines, immune checkpoint inhibitors). As an example, of 23 phase II/III clinical trials testing cancer vaccines, 78% failed [23]. Besides these rather discouraging figures, the financial costs for translational research are huge. The cost for successful translational development resulting in an approved novel drug exceeds US $ 1 billion [24, 25].
In view of these facts and circumstances the shortage of novel drugs for GBM might not be surprising.
The results from this in vitro study show that GuaDex, a cationic polymer with guanidine side-groups, has a potent cytotoxic effect on glioma cells including glioma cell spheroids. The mode of action appears very similar to that of tumor treating fields (TTS) affecting the intrinsic electrostatic properties of the tumor cells and like this disrupting their integrity [26].
The effect was observed at low µM concentrations (IC50 < ~ 3 µM) in contrast to the positive control (TMZ) that only showed a slight cytotoxic effect even at mM concentration. TMZ was dissolved in DMSO (dimethyl sulfoxide), an amphiphilic solvent. DMSO is frequently used in cell biology as enhancer of cell membrane penetration [27]. DMSO had by itself a slight cytotoxic effect (Fig. 3) and the difference to TMZ dissolved in DMSO was small.
In view of GuaDex significant in vitro GBM toxicity, the question is how it might be applied clinically for GBM therapy. GuaDex might not seem suitable for systemic administration because of BBB restriction owing to its chemical properties (size, charge etc.). Local application as an interstitial therapy might be feasible in connection with initial surgery of unifocal GBM. Local delivery of chemotherapeutic agents has been explored previously. Biodegradable wafers (Gliadel) with adsorbed carmustine implanted in connection with surgery have been studied in several human trials. Carmustine is a nitrosourea DNA alkylator. The wafers release the carmustine during a period of several weeks, i.e. a slow-release formulation. There appears to be some survival benefit for certain patient groups receiving this interstitial therapy [28]. The most common side effects with Gliadel implants include brain edema with convulsions, hemiparesis, aphasia, infection, and visual field defects [29]. Since in most cases, GBM recurrence seems to occur in the wall of the resection cavity within 20 mm of its margin, interstitial therapy appears to be attractive and an opportunity to prevent recurrence [30]. GuaDex could be adsorbed into a biodegradable/biocompatible matrix, implanted after surgery, and then it would gradually diffuse into the surrounding interstitial space. Although the concept of interstitial chemotherapy appears attractive and strait forward, there are several circumstances that may hamper successful targeting of remaining infiltrating tumor cells post-surgery. Increased interstitial pressure due to edema resulting from the tumor itself, as result of the surgery and maybe aggravated by the implant, can make efficient diffusion difficult to achieve. Furthermore, the anatomy of the brain interstitial space is tortuous, long and complex with hindrances to diffusion e.g., local limits ("dead ends") and local viscosity [31, 32] and, changes because of the GBM pathology. In spite of these examples of unfavourable factors that may hinder diffusion, GuaDex chemical properties might be an advantage, namely its strong cationic electrostatic charge, anti-microbial properties [33] and its molecular polydispersity. When GuaDex is in the interstitial space, there appears to be good possibilities for charge specific targeting of the electronegative GBM cells with subsequent tumor cell toxicity. This study indicates that the glioma electronegativity i.e., cell surface hypersialylation, contribute to the efficacy of GuaDex. Tumor cells with high sialyl expression and/or positive intracellular GFAP expression yield faster induction of GuaDex toxicity. Glial fibrillary acidic protein, GFAP, is thought to help to maintain astrocyte mechanical strength as well as the shape of cells, but its exact function remains poorly understood [34].
The feasibility of targeting electronegative tumor cells with a cationic polymer has been confirmed before [8]. Polymers with different charge were delivered into the urinary bladder through instillation on patients with superficial bladder cancer. The result showed very high tumor accumulation of cationic polymers and low uptake in normal tissue [8].
GuaDex polydispersity i.e., consisting of a cocktail of multiple molecular species, ranging from low molecular weight (mw) to higher mw, might facilitate diffusion/penetration into the heterogeneous and complex interstitial space. In addition, its apparent anti-microbial properties might decrease the risk of causing infection during the instillation [33].
In summary. In order to translate and test GuaDex in vitro efficacy into clinical studies, briefly, further pre-clinical studies need to be done, in particular determination of the safety i.e., possible toxicity of intra CNS instillation and how optimal diffusion into the surrounding interstitial space should be achieved. Development of a suitable biocompatible/degradable absorbent needs to be accomplished. Fast advancement to Phase 0 trials [35] would be desirable due to the limited relevance of pre-clinical in vivo models.
References
Hanif F, Muzaffar K, Perveen K et al (2017) Glioblastoma Multiforme: A review of its epidemiology and pathogenesis through clinical presentation and treatment. Asian Pac J Cancer Prev 18(1):3–9
Lara-Velazquez M, Al-Kharboosh R, Jeanneret S et al (2017) Advances in brain tumor surgery for glioblastoma in adults. Brain Sci 7(12):166
Sharif Y, Jumah F, Coplan L et al (2018) Blood brain barrier: a review of its anatomy and physiology in health and disease. Clin Anat 31(6):812–823
Dréan A, Goldwirt L, Verreault M et al (2016) Blood-brain barrier, cytotoxic chemotherapies and Glioblastoma. Expert Rev Neurother 16(11):1285–1300
Newlands ES, Stevens MF, Wedge SR et al (1997) Temozolomide: a review of its discovery, chemical properties, pre-clinical development and clinical trials. Cancer Treat Rev 23(1):35–61
Büll C, Stoel MA, den Brok MH et al (2014) Sialic acids sweeten a tumor’s life. Cancer Res 74(12):3199–3204
Fonseca LM, Silva VA, Freire-de-Lima L et al (2016) Glycosylation in cancer: interplay between multidrug resistance and epithelial-to-mesenchymal transition. Front Oncol 6:158
Holmberg AR, Wilchek M, Márquez M et al (1999) Ion exchange tumor targeting: A new approach. Clin Cancer Res 5:3056s–3058s
Meurling L, Márquez M, Nilsson S et al (2009) Polymer-conjugated guanidine is a potentially useful anti-tumor agent. Int J Oncol 35:281–285
Kroemer G, Pouyssegur J (2008) Tumor cell metabolism. Cancer Cell 13(6):472–482
Nowotarski SL, Woster PM, Casero RA Jr (2013) Polyamines and cancer: Implications for chemoprevention and chemotherapy. Expert Rev Mol Med 15:e3. https://doi.org/10.1017/erm.2013.3
Larsson R, Nygren P (1989) A rapid fluorometric method for semi-automated determination of cytotoxicity and cellular proliferation of human tumor cell lines in microculture. Anticancer Res 9:1111–1119
Piccinini F, Tesei A, Arienti C et al (2017) Cell counting and viability assessment of 2D and 3D cell cultures: excepted reliability of the trypan blue assay. Biol Proced Online 19:8. https://doi.org/10.1186/s12575-017-0056-3
Sirenko O, Mitlo T, Hesley J et al (2015) High-content assays for characterizing the viability and morphology of 3D cancer spheroids cultures. Assay Drug Dev Technol 13(7):402–414
Singh M, Close DA, Mukundan S et al (2015) Production of uniform 3D microtumors in hydrogel microwell arrays for measurement of viability, morphology, and signaling pathway activation. Assay Drug Dev Technol 13(9):570–583
Cai X, Sughrue ME (2018) Glioblastoma: new therapeutic strategies to address cellular and genomic complexity. Oncotarget 9(10):9540–9554
De Felice F, Musio D, Cassese R, Gravina GL, Tombolini V (2017) New approaches in Glioblastoma multiforme; the potential role of immune-check point inhibitors. Curr Cancer Drug Targets 17(3):282–289
Ozdemir-Kaynak E, Qutub AA, Yesil-Celiktas O (2018) Advances in Glioblastoma multiforme treatment: new models for nanoparticle therapy. Front Physiol 9:170. https://doi.org/10.3389/fphys.2018.00170
Kirson ED, Gurvich Z, Schneiderman R et al (2004) Disruption of cancer cell replication by alternating electric fields. Cancer Res 64:3288–3295
Sarkaria JN, Hu LS, Parney IF et al (2018) Is the blood–brain barrier really disrupted in all glioblastomas? A critical assessment of existing clinical data. Neuro-Oncol 20(2):184–191
Butler D (2008) Translational research: crossing the valley of death. Nature 453(7197):840–842
Mak IWY, Evaniew N, Ghert M (2014) Lost in translation: animal models and clinical trials in cancer treatment. Am J Transl Res 6(2):114–118
Ogi C, Aruga A (2013) Immunological monitoring of anticancer vaccines in clinical trials. Oncoimmunology 2(8):e26012. https://doi.org/10.4161/onci.26012
DiMasi JA, Grabowski HG, Hansen RA (2016) Innovation in the pharmaceutical industry: new estimates of R&D costs. J Health Econ 47:20–33
Munoz C (2014) The price of failure. The Economist. https://www.economist.com/business/2014/11/27/the-price-of-failure. Accessed 15 Feb 2021
Branter J, Basu S, Smith S (2018) Tumour treating fields in a combinational therapeutic approach. Oncotarget 9(93):36631–36644
Gurtovenko AA, Anwar J (2007) Modulating the structure and properties of cell membranes: the molecular mechanism of action of dimethyl sulfoxide. J Phys Chem 111(35):10453–10460
Chaichana KL, Zaidi H, Pendleton C et al (2011) The efficacy of carmustine wafers for older patients with Glioblastoma multiforme: prolonging survival. Neurol Res 33(7):759–764
Perry J, Chambers A, Spithoff K et al (2007) Gliadel wafers in the treatment of malignant glioma: a systematic review. Curr Oncol 14(5):189–194
De Bonis P, Anile C, Pompucci A et al (2013) The influence of surgery on recurrence pattern of glioblastoma. Clin Neurol Neurosurg 115(1):37–43
Nicholson C (2001) Diffusion and related transport mechanisms in brain tissue. Rep Prog Phys 64(7):815–884
Nicholson C, Hrabětová S (2017) Brain extracellular space: the final frontier of neuroscience. Biophys J 113(10):2133–2142
Escamilla-García E, Alcázar-Pizaña AG, Segoviano-Ramírez JC et al (2017) Antimicrobial activity of a cationic guanidine compound against two pathogenic oral bacteria. Int J Microbiol 2017:5924717
Li D, Liu X, Liu T et al (2020) Neurochemical regulation of the expression and function of glial fibrillary acidic protein in astrocytes. Glia 68(5):878–897
Kummar S, Rubinstein L, Kinders R et al (2008) Phase 0 clinical trials: conceptions and misconceptions. Cancer J 14(3):133–137
Westermark B, Pontén J, Hugosson R (1973) Determinants for the establishment of permanent tissue culture lines from human gliomas. Acta Path Microbiol Scand 81(6):791–805
Westermark B (1973) The deficient density-dependent growth control of human malignant glioma cells and virus-transformed glia-like cells in culture. Int J Cancer 12(2):438–451
Bongcam-Rudloff E, Nistér M, Betsholtz C, Wang JL, Stenman G, Huebner K et al (1991) Human glial fibrillary acidic protein: complementary DNA cloning, chromosome localization, and messenger RNA expression in human glioma cell lines of various phenotypes. Cancer Res 51:1553–1560
Bigner DD, Bigner SH, Pontén J et al (1981) Heterogeneity of Genotypic and phenotypic characteristics of fifteen permanent cell lines derived from human gliomas. J Neuropath Exp Neurol 40(3):201–229
Nistér M, Libermmann TA, Betsholtz C et al (1988) Expression of messenger RNAs for platelet-derived growth factor and transforming growth factor-alpha and their receptors in human malignant glioma cell lines. Cancer Res 48:3910–3918
Nistér M, Heldin CH, Westermark, (1986) Clonal variation in the production of a platelet-derived growth factor-like protein and expression of corresponding receptors in a human malignant glioma. Cancer Res 46:332–340
Mehta S, Huillard E, Kesari S et al (2011) The central nervous system-restricted transcription factor olig2 opposes p53 responses to genotoxic damage in neural progenitors and malignant glioma. Cancer Cell 19(3):359–371
Holmberg Olausson K, Maire CL, Haidar S, Ling J et al (2014) Prominin-1 (CD133) defines both stem and non-stem cell populations in CNS development and gliomas. PLoS ONE 9(9):e106694. https://doi.org/10.1371/journal.pone.0106694
Stevens MM, Maire CL, Chou N, Murakami MA, Knoff DS, Kikuchi Y et al (2016) Drug sensitivity of single cancer cells in predicted by changes in mass accumulation rate. Nat Biotech 34(11):1161–1167
Acknowledgements
Supported by grants from The Cancer Society in Stockholm and The King Gustav V Jubilee Fund.
Funding
Open access funding provided by Karolinska Institute. Supported by grants from The Cancer Society in Stockholm and The King Gustav V Jubilee Fund.
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Contributions
All the authors were involved in study design, MM and KHO performed the lab work and prepared a manuscript draft based on analysis done by all authors; AA data analysis, ARH and LM prepared GuaDex conjugate, SN and ARH prepared final manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable. See Informed consent.
Authors' information
Not special remarks.
Research involvi.ng human participants and/or animals
Not applicable.
Informed consent
Not applicable.
Competing interest
MM, KHO and AA declare they have no conflict of interests. ARH and LM are the inventors of GuaDex, ARH and SN are board members of DexTech Medical, the owner of the GuaDex patent.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Márquez, M., Olausson, K.H., Alaiya, A. et al. Poly-guanidine shows high cytotoxicity in glioma cell cultures and glioma stem cells. Invest New Drugs 40, 565–575 (2022). https://doi.org/10.1007/s10637-022-01233-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-022-01233-7