Summary
Objective The problem of drug resistance to BRAF-targeted therapy often occurs in melanoma treatment. Activation of PI3K/AKT/mTOR signaling pathway is one of the mechanisms of acquired resistance and a potential target for treatment. In the current research, we investigated that dual inhibition of mTOR and MEK synergistically reduced the viability of melanoma cells in vitro. Methods A combination of rapamycin (a macrolide immunosuppressant, mTOR inhibitor) and binimetinib (an anti-cancer small molecule, selective inhibitor of MEK) was studied using a panel of melanoma cell lines, including patient-derived cells. Results It was found, that combinatorial therapy of rapamycin (250 nM) and binimetinib (2 μM) resulted in 25% of cell viability compared to either rapamycin (85%) or binimetinib alone (50%) for A375 and vemurafenib-resistant Mel IL/R cells. The suppressed activation of mTOR and MEK by combined rapamycin and binimetinib treatment was confirmed using Western blot assay. Cell death occured via the apoptosis pathway; however, the combination treatment significantly increased the apoptosis only for Mel IL/R cells. The enhanced cytotoxic effect was also associated with enhanced cell cycle arrest in the G0/G1 phase. Conclusion In general, we provide the evidence that dual inhibition of mTOR and MEK could be promising for further preclinical investigations.







Similar content being viewed by others
Data availability
All data and results contained in this paper are available upon request.
References
Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, Dummer R, Garbe C, Testori A, Maio M, Hogg D, Lorigan P, Lebbe C, Jouary T, Schadendorf D, Ribas A, O'Day SJ, Sosman JA, Kirkwood JM, Eggermont AM, Dreno B, Nolop K, Li J, Nelson B, Hou J, Lee RJ, Flaherty KT, McArthur G, BRIM-3 Study Group (2011) Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med 364:2507–2516. https://doi.org/10.1056/NEJMoa1103782
McCubrey JA, Steelman LS, Kempf CR et al (2011) Therapeutic resistance resulting from mutations in Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR signaling pathways. J Cell Physiol 226:2762–2781. https://doi.org/10.1002/jcp.22647
Calero R, Morchon E, Martinez-Argudo I, Serrano R (2017) Synergistic anti-tumor effect of 17AAG with the PI3K/mTOR inhibitor NVP-BEZ235 on human melanoma. Cancer Lett 406:1–11. https://doi.org/10.1016/j.canlet.2017.07.021
Chappell WH, Steelman LS, Long JM, et al (2011) Ras/Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR inhibitors: rationale and importance to inhibiting these pathways in human health. Oncotarget 2:135–164. https://doi.org/10.18632/oncotarget.240
Wang A-X, Qi X-Y (2013) Targeting RAS/RAF/MEK/ERK signaling in metastatic melanoma. IUBMB Life 65:748–758. https://doi.org/10.1002/iub.1193
Turke AB, Song Y, Costa C, Cook R, Arteaga CL, Asara JM, Engelman JA (2012) MEK inhibition leads to PI3K/AKT activation by relieving a negative feedback on ERBB receptors. Cancer Res 72:3228–3237. https://doi.org/10.1158/0008-5472.CAN-11-3747
Kim KB, Kefford R, Pavlick AC, Infante JR, Ribas A, Sosman JA, Fecher LA, Millward M, McArthur GA, Hwu P, Gonzalez R, Ott PA, Long GV, Gardner OS, Ouellet D, Xu Y, DeMarini DJ, le NT, Patel K, Lewis KD (2013) Phase II study of the MEK1/MEK2 inhibitor Trametinib in patients with metastatic BRAF-mutant cutaneous melanoma previously treated with or without a BRAF inhibitor. J Clin Oncol Off J Am Soc Clin Oncol 31:482–489. https://doi.org/10.1200/JCO.2012.43.5966
Ascierto PA, Schadendorf D, Berking C, Agarwala SS, van Herpen CML, Queirolo P, Blank CU, Hauschild A, Beck JT, St-Pierre A, Niazi F, Wandel S, Peters M, Zubel A, Dummer R (2013) MEK162 for patients with advanced melanoma harbouring NRAS or Val600 BRAF mutations: a non-randomised, open-label phase 2 study. Lancet Oncol 14:249–256. https://doi.org/10.1016/S1470-2045(13)70024-X
Dummer R, Schadendorf D, Ascierto PA, Arance A, Dutriaux C, di Giacomo AM, Rutkowski P, del Vecchio M, Gutzmer R, Mandala M, Thomas L, Demidov L, Garbe C, Hogg D, Liszkay G, Queirolo P, Wasserman E, Ford J, Weill M, Sirulnik LA, Jehl V, Bozón V, Long GV, Flaherty K (2017) Binimetinib versus dacarbazine in patients with advanced NRAS-mutant melanoma (NEMO): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol 18:435–445. https://doi.org/10.1016/S1470-2045(17)30180-8
Kopetz S, Desai J, Chan E, Hecht JR, O'Dwyer PJ, Maru D, Morris V, Janku F, Dasari A, Chung W, Issa JPJ, Gibbs P, James B, Powis G, Nolop KB, Bhattacharya S, Saltz L (2015) Phase II pilot study of Vemurafenib in patients with metastatic BRAF-mutated colorectal Cancer. J Clin Oncol 33:4032–4038. https://doi.org/10.1200/JCO.2015.63.2497
Matulonis U, Vergote I, Backes F, Martin LP, McMeekin S, Birrer M, Campana F, Xu Y, Egile C, Ghamande S (2015) Phase II study of the PI3K inhibitor pilaralisib (SAR245408; XL147) in patients with advanced or recurrent endometrial carcinoma. Gynecol Oncol 136:246–253. https://doi.org/10.1016/j.ygyno.2014.12.019
Jokinen E, Koivunen JP (2015) MEK and PI3K inhibition in solid tumors: rationale and evidence to date. Ther Adv Med Oncol 7:170–180. https://doi.org/10.1177/1758834015571111
Carracedo A, Ma L, Teruya-Feldstein J, Rojo F, Salmena L, Alimonti A, Egia A, Sasaki AT, Thomas G, Kozma SC, Papa A, Nardella C, Cantley LC, Baselga J, Pandolfi PP (2008) Inhibition of mTORC1 leads to MAPK pathway activation through a PI3K-dependent feedback loop in human cancer. J Clin Invest 118:3065–3074. https://doi.org/10.1172/JCI34739
Sweetlove M, Wrightson E, Kolekar S, Rewcastle GW, Baguley BC, Shepherd PR, Jamieson SMF (2015) Inhibitors of pan-PI3K signaling synergize with BRAF or MEK inhibitors to prevent BRAF-mutant melanoma cell growth. Front Oncol 5. https://doi.org/10.3389/fonc.2015.00135
Rewcastle GW, Kolekar S, Buchanan CM, et al (2017) Biological characterization of SN32976, a selective inhibitor of PI3κ and mTOR with preferential activity to PI3κα, in comparison to established pan PI3κ inhibitors. Oncotarget. https://doi.org/10.18632/oncotarget.17730
Leung EY, Askarian-Amiri M, Finlay GJ, Rewcastle GW, Baguley BC (2015) Potentiation of growth inhibitory responses of the mtor inhibitor everolimus by dual mTORC1/2 inhibitors in cultured breast cancer cell lines. PLoS One 10. https://doi.org/10.1371/journal.pone.0131400
Seto B (2012) Rapamycin and mTOR: a serendipitous discovery and implications for breast cancer. Clin Transl Med 1:29. https://doi.org/10.1186/2001-1326-1-29
Weekes CD, Von Hoff DD, Adjei AA et al (2013) Multicenter phase I trial of the mitogen-activated protein kinase 1/2 inhibitor BAY 86-9766 in patients with advanced cancer. Clin Cancer Res An Off J Am Assoc Cancer Res 19:1232–1243. https://doi.org/10.1158/1078-0432.CCR-12-3529
Carlino MS, Gowrishankar K, Saunders CAB, Pupo GM, Snoyman S, Zhang XD, Saw R, Becker TM, Kefford RF, Long GV, Rizos H (2013) Antiproliferative effects of continued mitogen-activated protein kinase pathway inhibition following acquired resistance to BRAF and/or MEK inhibition in melanoma. Mol Cancer Ther 12:1332–1342. https://doi.org/10.1158/1535-7163.MCT-13-0011
Carlino MS, Todd JR, Gowrishankar K, Mijatov B, Pupo GM, Fung C, Snoyman S, Hersey P, Long GV, Kefford RF, Rizos H (2014) Differential activity of MEK and ERK inhibitors in BRAF inhibitor resistant melanoma. Mol Oncol 8:544–554. https://doi.org/10.1016/j.molonc.2014.01.003
Egorov EE, Moldaver MV, Vishniakova KS et al (2007) Enhanced control of proliferation in telomerized cells. Ontogenez
Emelyanova M, Ghukasyan L, Abramov I, et al (2017) Detection of BRAF, NRAS, KIT, GNAQ, GNA11 and MAP2K1/2 mutations in Russian melanoma patients using LNA PCR clamp and biochip analysis. Oncotarget 8:52304–52320. https://doi.org/10.18632/oncotarget.17014
Chou TC, Talalay P (1984) Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzym Regul 22:27–55
Friedrich J, Seidel C, Ebner R, Kunz-Schughart LA (2009) Spheroid-based drug screen: considerations and practical approach. Nat Protoc 4:309–324. https://doi.org/10.1038/nprot.2008.226
Mikhaĭlova IN, Lukashina MI, Baryshnikov AI, et al (2005) [melanoma cell lines as the basis for antitumor vaccine preparation]. Vestn Ross Akad Meditsinskikh Nauk 37–40
Ryabaya O, Prokofieva A, Akasov R, Khochenkov D, Emelyanova M, Burov S, Markvicheva E, Inshakov A, Stepanova E (2019) Metformin increases antitumor activity of MEK inhibitor binimetinib in 2D and 3D models of human metastatic melanoma cells. Biomed Pharmacother 109:2548–2560. https://doi.org/10.1016/j.biopha.2018.11.109
Shi H, Kong X, Ribas A, Lo RS (2011) Combinatorial treatments that overcome PDGFRβ-driven resistance of melanoma cells to V600EB-RAF inhibition. Cancer Res 71:5067–5074. https://doi.org/10.1158/0008-5472.CAN-11-0140
Kircher DA, Silvis MR, Cho JH, Holmen SL (2016) Melanoma brain metastasis: mechanisms, models, and medicine. Int J Mol Sci 17. https://doi.org/10.3390/ijms17091468
Rao RD, Windschitl HE, Allred JB, Lowe VJ, Maples WJ, Gornet MK, Suman VJ, Creagan ET, Pitot HC, Markovic SN (2006) Phase II trial of the mTOR inhibitor everolimus (RAD-001) in metastatic melanoma. J Clin Oncol 24:8043–8043. https://doi.org/10.1200/jco.2006.24.18_suppl.8043
Kiessling MK, Curioni-Fontecedro A, Samaras P, Lang S, Scharl M, Aguzzi A, Oldrige DA, Maris JM, Rogler G (2016) Targeting the mTOR complex by Everolimus in NRAS mutant Neuroblastoma. PLoS One 11:e0147682. https://doi.org/10.1371/journal.pone.0147682
Juric D, Soria J-C, Sharma S, Banerji U, Azaro A, Desai J, Ringeisen FP, Kaag A, Radhakrishnan R, Hourcade-Potelleret F, Maacke H, Rodon Ahnert J (2014) A phase 1b dose-escalation study of BYL719 plus binimetinib (MEK162) in patients with selected advanced solid tumors. J Clin Oncol 32:9051–9051. https://doi.org/10.1200/jco.2014.32.15_suppl.9051
Haagensen EJ, Kyle S, Beale GS, Maxwell RJ, Newell DR (2012) The synergistic interaction of MEK and PI3K inhibitors is modulated by mTOR inhibition. Br J Cancer 106:1386–1394. https://doi.org/10.1038/bjc.2012.70
Zimmerman MA, Biggers CD, Li PA (2018) Rapamycin treatment increases hippocampal cell viability in an mTOR-independent manner during exposure to hypoxia mimetic, cobalt chloride. BMC Neurosci 19:82. https://doi.org/10.1186/s12868-018-0482-4
Stefanovska B, Vicier CE, Dayris T, Ogryzko V, Scott V, Bouakka I, Delaloge S, Rocca A, le Saux O, Trédan O, Bachelot T, André F, Fromigué O (2020) Rapalog-mediated repression of Tribbles Pseudokinase 3 regulates pre-mRNA splicing. Cancer Res 80:2190–2203. https://doi.org/10.1158/0008-5472.CAN-19-2366
Wu YL, Maachani UB, Schweitzer M, Singh R, Wang M, Chang R, Souweidane MM (2017) Dual inhibition of PI3K/AKT and MEK/ERK pathways induces synergistic antitumor effects in diffuse intrinsic Pontine Glioma cells. Transl Oncol 10:221–228. https://doi.org/10.1016/j.tranon.2016.12.008
Wan X, Harkavy B, Shen N, Grohar PHL (2007) Rapamycin induces feedback activation of Akt signaling through an IGF-1R-dependent mechanism. Oncogene 26:1932–1940. https://doi.org/10.1038/sj.onc.1209990
Shi Y (2005) Mammalian target of rapamycin inhibitors activate the AKT kinase in multiple myeloma cells by up-regulating the insulin-like growth factor receptor/insulin receptor substrate-1/phosphatidylinositol 3-kinase cascade. Mol Cancer Ther 4:1533–1540. https://doi.org/10.1158/1535-7163.MCT-05-0068
Pitts TM, Newton TP, Bradshaw-Pierce EL, Addison R, Arcaroli JJ, Klauck PJ, Bagby SM, Hyatt SL, Purkey A, Tentler JJ, Tan AC, Messersmith WA, Eckhardt SG, Leong S (2014) Dual pharmacological targeting of the MAP kinase and PI3K/mTOR pathway in preclinical models of colorectal Cancer. PLoS One 9:e113037. https://doi.org/10.1371/journal.pone.0113037
Bedard PL, Tabernero J, Janku F, Wainberg ZA, Paz-Ares L, Vansteenkiste J, van Cutsem E, Pérez-García J, Stathis A, Britten CD, le N, Carter K, Demanse D, Csonka D, Peters M, Zubel A, Nauwelaerts H, Sessa C (2015) A phase Ib dose-escalation study of the oral pan-PI3K inhibitor buparlisib (BKM120) in combination with the oral MEK1/2 inhibitor trametinib (GSK1120212) in patients with selected advanced solid tumors. Clin Cancer Res An Off J Am Assoc Cancer Res 21:730–738. https://doi.org/10.1158/1078-0432.CCR-14-1814
Funding
This work was supported by the Russian Science Foundation under Grant 14–35–00107.
Author information
Authors and Affiliations
Contributions
OR, AR, AP, DK and AI performed experiments, acquired and analysed the data. OR and AP designed the study, OR and AR wrote the manuscript. NS revised the manuscript. IA and DK contributed to materials and tools. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
This article does not contain any studies with human participants or animals performed by any of the authors. The protocol to obtain Mel IL cells was initially approved by the N.N. Blokhin National Medical Research Center ethics committee in 1999 with written consent. Since then, the cell line was patented (#RU 2287577 C1).
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare that there are no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
A.Supplementary Information
Supplementary file 1
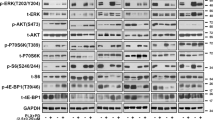
CI index calculations at a consistent ratio of binimetinib (2 μM) and rapamycin (250 nM). The output of the Chou-Talalay method, including Dm, m, and r with CI at the ED50, ED75, and ED90 values. Supplementary file 2 The effect of rapamycin, binimetinib, and its combination on normal cell proliferation. Cell viability with rapamycin (250 nM) and binimetinib (2 μM) was determined in keratinocytes HaCaT and hFB-hTERT6 skin fibroblasts by MTT assay for 48 h. Data are expressed as mean ± SD of at least three independent experiments done in triplicate. One-way ANOVA followed by the post hoc Duncan’s test (hFB-hTERT6 F2,6 = 29.42, p = 0.0008; HaCaT F2,6 = 33.43, p = 0.0006). Supplementary data 3 Combined treatment with rapamycin and binimetinib decrease the expression of mTOR and MEK but not AKT. The unprocessed data for Figs. 3. Supplementary file 4 The enhancement of apoptosis by combined rapamycin and binimetinib in melanoma cells. Apoptosis was measured by annexin V/Propidium iodate staining in Mel IL, Mel IL/R and A375 cells after 24 h and 48 h treatment with rapamycin (250 nM), binimetinib (2 μM) or both. Values are percentage increase to untreated control. The data represents 3 individual experiments, p < 0.05 vs. Control. Supplementary file 5 The enhancement of apoptosis by combined rapamycin and binimetinib in melanoma cells (caspase 3/7 staining). Caspase 3 and caspase 7 activities were measured in Mel IL, Mel IL/R, and A375 cells with rapamycin (250 nM), binimetinib (2 μM), or their combination after 4 h treatment. Data are presented as mean amount of stained cells (%) to untreated control (non-stained cells). Left peak (light grey) means control stained cells to either caspase 3 or caspase 7, right peak (dark grey) means experimental group results for caspase 3 or caspase 7. Supplementary file 6 Cell cycle distribution under rapamycin and binimetinib treatment. Mel IL, Mel IL/R, and A375 cells were treated with binimetinib (2 μM), rapamycin (250 nM), or their combination for 24 h followed by propidium iodide staining and flow cytometry analysis. The histogram represents the data from three independent experiments, CV 5%. (PDF 1622 kb)
Rights and permissions
About this article
Cite this article
Ryabaya, O.O., Abramov, I.S., Khochenkov, D.A. et al. Rapamycin synergizes the cytotoxic effects of MEK inhibitor binimetinib and overcomes acquired resistance to therapy in melanoma cell lines in vitro. Invest New Drugs 39, 987–1000 (2021). https://doi.org/10.1007/s10637-021-01089-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-021-01089-3