Summary
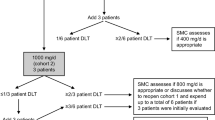
Background Tumor-associated macrophages (TAMs) promote tumor growth, metastasis, and therapeutic resistance via colony-stimulating factor-1 (CSF-1), acting through CSF-1 receptor (CSF-1R) signaling. This phase 1 study determined the safety, tolerability, pharmacokinetics-pharmacodynamics, immunogenicity, and efficacy of the anti–CSF-1R antibody LY3022855 in solid tumors. Methods Patients with advanced solid tumors refractory to standard therapy were enrolled and treated in 2 dosing cohorts: weight-based (part A) and non–weight-based (part B). Part A patients were assigned to intravenous (IV) dose-escalation cohorts: 2.5 mg/kg once per week (QW), 0.3 mg/kg QW, 0.6 mg/kg QW, 1.25 mg/kg once every 2 weeks (Q2W) and 1.25 mg/kg QW doses of LY3022855. Non–weight-based doses in part B were 100 mg and 150 mg IV QW. Results Fifty-two patients (mean age 58.6 ± 10.4 years) were treated with ≥1 dose of LY3022855 (range: 4–6). Five dose-limiting toxicities (left ventricular dysfunction, anemia, pancreatitis, rhabdomyolysis, and acute kidney injury) occurred in 4 patients. The non–weight-based 100 mg QW dose was established as the RP2D. The most common treatment-emergent adverse events were increase in liver function variables, fatigue, nausea, vomiting, diarrhea, anorexia, pyrexia, increased lipase, amylase, and lactate dehydrogenase. Clearance decreased with increasing dose and weight-based dosing had minimal effect on pharmacokinetics. Serum CSF-1, and IL-34 levels increased at higher doses and more frequent dosing, whereas TAMs and CD14dimCD16bright levels decreased. Three patients achieved stable disease. No responses were seen. Conclusions LY3022855 was well tolerated and showed dose-dependent pharmacokinetics-pharmacodynamics and limited clinical activity in a heterogenous solid tumor population. ClinicalTrials.gov ID NCT01346358 (Registration Date: May 3, 2011).



Similar content being viewed by others
Data availability
Lilly provides access to all individual participant data collected during the trial, after anonymization, with the exception of pharmacokinetic or genetic data. Data are available to request 6 months after the indication studied has been approved in the US and EU and after primary publication acceptance, whichever is later. No expiration date of data requests is currently set once they are made available. Access is provided after a proposal has been approved by an independent review committee identified for this purpose and after receipt of a signed data sharing agreement. Data and documents, including the study protocol, statistical analysis plan, clinical study report, and blank or annotated case report forms, will be provided in a secure data-sharing environment for up to 2 years per proposal. For details on submitting a request, see the instructions provided at www.clinicalstudydatarequest.com.
Code availability
Not applicable.
References
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144(5):646–674. https://doi.org/10.1016/j.cell.2011.02.013
Cassetta L, Kitamura T (2018) Targeting tumor-associated macrophages as a potential strategy to enhance the response to immune checkpoint inhibitors. Front Cell Dev Biol 6:38. https://doi.org/10.3389/fcell.2018.00038
Papadopoulos KP, Gluck L, Martin LP, Olszanski AJ, Tolcher AW, Ngarmchamnanrith G, Rasmussen E, Amore BM, Nagorsen D, Hill JS, Stephenson J Jr (2017) First-in-human study of AMG 820, a monoclonal anti-Colony-stimulating factor 1 receptor antibody, in patients with advanced solid tumors. Clin Cancer Res 23(19):5703–5710. https://doi.org/10.1158/1078-0432.CCR-16-3261
Cannarile MA, Weisser M, Jacob W, Jegg AM, Ries CH, Ruttinger D (2017) Colony-stimulating factor 1 receptor (CSF1R) inhibitors in cancer therapy. J Immunother Cancer 5(1):53. https://doi.org/10.1186/s40425-017-0257-y
Lin H, Lee E, Hestir K, Leo C, Huang M, Bosch E, Halenbeck R, Wu G, Zhou A, Behrens D, Hollenbaugh D, Linnemann T, Qin M, Wong J, Chu K, Doberstein SK, Williams LT (2008) Discovery of a cytokine and its receptor by functional screening of the extracellular proteome. Science 320(5877):807–811. https://doi.org/10.1126/science.1154370
Pixley FJ, Stanley ER (2004) CSF-1 regulation of the wandering macrophage: complexity in action. Trends Cell Biol 14(11):628–638. https://doi.org/10.1016/j.tcb.2004.09.016
DeNardo DG, Brennan DJ, Rexhepaj E, Ruffell B, Shiao SL, Madden SF, Gallagher WM, Wadhwani N, Keil SD, Junaid SA, Rugo HS, Hwang ES, Jirstrom K, West BL, Coussens LM (2011) Leukocyte complexity predicts breast cancer survival and functionally regulates response to chemotherapy. Cancer Discov 1(1):54–67. https://doi.org/10.1158/2159-8274.CD-10-0028
Subimerb C, Pinlaor S, Lulitanond V, Khuntikeo N, Okada S, McGrath MS, Wongkham S (2010) Circulating CD14(+) CD16(+) monocyte levels predict tissue invasive character of cholangiocarcinoma. Clin Exp Immunol 161(3):471–479. https://doi.org/10.1111/j.1365-2249.2010.04200.x
Zhu Y, Knolhoff BL, Meyer MA, Nywening TM, West BL, Luo J, Wang-Gillam A, Goedegebuure SP, Linehan DC, DeNardo DG (2014) CSF1/CSF1R blockade reprograms tumor-infiltrating macrophages and improves response to T-cell checkpoint immunotherapy in pancreatic cancer models. Cancer Res 74(18):5057–5069. https://doi.org/10.1158/0008-5472.CAN-13-3723
ClinicalTrials.Gov. A Study of ARRY-382 in Patients With Selected Advanced or Metastatic Cancers. Available on https://clinicaltrials.gov/ct2/show/NCT01316822. Accessed on 5 May 2020
ClinicalTrials.gov. Phase 1 Study of PLX7486 as Single Agent in Patients With Advanced Solid Tumors. Available on https://clinicaltrials.gov/ct2/show/NCT01804530. Accessed on April 4 2020
ClinicalTrials.gov. Phase I/II Study of BLZ945 Single Agent or BLZ945 in Combination With PDR001 in Advanced Solid Tumors. Available on https://clinicaltrials.gov/ct2/show/NCT02829723. Accessed on April 4 2020
Gomez-Roca CA, Italiano A, Le Tourneau C, Cassier PA, Toulmonde M, D'Angelo SP, Campone M, Weber KL, Loirat D, Cannarile MA, Jegg AM, Ries C, Christen R, Meneses-Lorente G, Jacob W, Klaman I, Ooi CH, Watson C, Wonde K, Reis B, Michielin F, Ruttinger D, Delord JP, Blay JY (2019) Phase I study of emactuzumab single agent or in combination with paclitaxel in patients with advanced/metastatic solid tumors reveals depletion of immunosuppressive M2-like macrophages. Ann Oncol 30(8):1381–1392. https://doi.org/10.1093/annonc/mdz163
ClinicalTrials.gov. A Study of Cabiralzumab Given by Itself or With Nivolumab in Advanced Cancer or Cancer That Has Spread. Available on https://clinicaltrials.gov/ct2/show/NCT03158272. Accessed on April 4 2020
ClinicalTrials.gov. Phase Ib/II Study of MCS110 in Combination With PDR001 in Patients With Advanced Malignancies. Available on https://clinicaltrials.gov/ct2/show/NCT02807844. Accessed on April 4 2020
Insight A IMC.CS4. Available on https://adisinsight.springer.com/drugs/800034411. Accessed 5 May 2020
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45(2):228–247. https://doi.org/10.1016/j.ejca.2008.10.026
U.S. Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0. Available on https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/Archive/CTCAE_4.0_2009-05-29_QuickReference_8.5x11.pdf. Accesssed 16 mar 2020
United States Food and Drug Administration. Immunogenicity Assessment for Therapeutic Protein Products. Available at https://www.fda.gov/media/85017/download. Accessed on October 1 2020
Dowlati A, Rugo H, Harvey D, Kudchadkar R, Carvajal R, Manji G, Hamid O, Klempner S, Tang S, Yu D, Kauh J, Schaer D, Tate S, Wesolowski R (2017) A phase I study of LY3022855, a colony-stimulating factor-1 receptor (CSF-1R) inhibitor, in patients (pts) with advanced solid tumors. J Clin Oncol 35:2523. https://doi.org/10.1200/JCO.2017.35.15_suppl.2523
Autio K, Klebanoff C, Schaer D, Kauh J, Slovin S, Blinder V, Comen E, Danila D, Hoffman D, Kang S, McAndrew P, Modi S, Morris M, Rathkopf D, Sanford R, Tate S, Yu D, McArthur H (2019) Phase 1 study of LY3022855, a colony-stimulating factor-1 receptor (CSF-1R) inhibitor, in patients with metastatic breast cancer (MBC) or metastatic castration-resistant prostate cancer (MCRPC). J Clin Oncol 37:2548. https://doi.org/10.1200/JCO.2019.37.15_suppl.2548
Lee JH, Chen TW, Hsu CH, Yen YH, Yang JC, Cheng AL, Sasaki SI, Chiu LL, Sugihara M, Ishizuka T, Oguma T, Tajima N, Lin CC (2020) A phase I study of pexidartinib, a colony-stimulating factor 1 receptor inhibitor, in Asian patients with advanced solid tumors. Investig New Drugs 38(1):99–110. https://doi.org/10.1007/s10637-019-00745-z
Wesolowski R, Sharma N, Reebel L, Rodal MB, Peck A, West BL, Marimuthu A, Severson P, Karlin DA, Dowlati A, Le MH, Coussens LM, Rugo HS (2019) Phase Ib study of the combination of pexidartinib (PLX3397), a CSF-1R inhibitor, and paclitaxel in patients with advanced solid tumors. Ther Adv Med Oncol 11:1758835919854238. https://doi.org/10.1177/1758835919854238
von Tresckow B, Morschhauser F, Ribrag V, Topp MS, Chien C, Seetharam S, Aquino R, Kotoulek S, de Boer CJ, Engert A (2015) An open-label, multicenter, phase I/II study of JNJ-40346527, a CSF-1R inhibitor, in patients with relapsed or refractory Hodgkin lymphoma. Clin Cancer Res 21(8):1843–1850. https://doi.org/10.1158/1078-0432.CCR-14-1845
Ovacik M, Lin K (2018) Tutorial on monoclonal antibody pharmacokinetics and its considerations in early development. Clin Transl Sci 11(6):540–552. https://doi.org/10.1111/cts.12567
Hendrikx J, Haanen J, Voest EE, Schellens JHM, Huitema ADR, Beijnen JH (2017) Fixed dosing of monoclonal antibodies in oncology. Oncologist 22(10):1212–1221. https://doi.org/10.1634/theoncologist.2017-0167
Bai S, Jorga K, Xin Y, Jin D, Zheng Y, Damico-Beyer LA, Gupta M, Tang M, Allison DE, Lu D, Zhang Y, Joshi A, Dresser MJ (2012) A guide to rational dosing of monoclonal antibodies. Clin Pharmacokinet 51(2):119–135. https://doi.org/10.2165/11596370-000000000-00000
Ries CH, Cannarile MA, Hoves S, Benz J, Wartha K, Runza V, Rey-Giraud F, Pradel LP, Feuerhake F, Klaman I, Jones T, Jucknischke U, Scheiblich S, Kaluza K, Gorr IH, Walz A, Abiraj K, Cassier PA, Sica A, Gomez-Roca C, de Visser KE, Italiano A, Le Tourneau C, Delord JP, Levitsky H, Blay JY, Ruttinger D (2014) Targeting tumor-associated macrophages with anti-CSF-1R antibody reveals a strategy for cancer therapy. Cancer Cell 25(6):846–859. https://doi.org/10.1016/j.ccr.2014.05.016
Pradel LP, Ooi CH, Romagnoli S, Cannarile MA, Sade H, Ruttinger D, Ries CH (2016) Macrophage susceptibility to Emactuzumab (RG7155) treatment. Mol Cancer Ther 15(12):3077–3086. https://doi.org/10.1158/1535-7163.MCT-16-0157
Kong LQ, Zhu XD, Xu HX, Zhang JB, Lu L, Wang WQ, Zhang QB, Wu WZ, Wang L, Fan J, Tang ZY, Sun HC (2013) The clinical significance of the CD163+ and CD68+ macrophages in patients with hepatocellular carcinoma. PLoS One 8(3):e59771. https://doi.org/10.1371/journal.pone.0059771
Medrek C, Ponten F, Jirstrom K, Leandersson K (2012) The presence of tumor associated macrophages in tumor stroma as a prognostic marker for breast cancer patients. BMC Cancer 12:306. https://doi.org/10.1186/1471-2407-12-306
Minami K, Hiwatashi K, Ueno S, Sakoda M, Iino S, Okumura H, Hashiguchi M, Kawasaki Y, Kurahara H, Mataki Y, Maemura K, Shinchi H, Natsugoe S (2018) Prognostic significance of CD68, CD163 and Folate receptor-beta positive macrophages in hepatocellular carcinoma. Exp Ther Med 15(5):4465–4476. https://doi.org/10.3892/etm.2018.5959
Ni C, Yang L, Xu Q, Yuan H, Wang W, Xia W, Gong D, Zhang W, Yu K (2019) CD68- and CD163-positive tumor infiltrating macrophages in non-metastatic breast cancer: a retrospective study and meta-analysis. J Cancer 10(19):4463–4472. https://doi.org/10.7150/jca.33914
Gyori D, Lim EL, Grant FM, Spensberger D, Roychoudhuri R, Shuttleworth SJ, Okkenhaug K, Stephens LR, Hawkins PT (2018) Compensation between CSF1R+ macrophages and Foxp3+ Treg cells drives resistance to tumor immunotherapy. JCI Insight 3(11). https://doi.org/10.1172/jci.insight.120631
Kumar V, Donthireddy L, Marvel D, Condamine T, Wang F, Lavilla-Alonso S, Hashimoto A, Vonteddu P, Behera R, Goins MA, Mulligan C, Nam B, Hockstein N, Denstman F, Shakamuri S, Speicher DW, Weeraratna AT, Chao T, Vonderheide RH, Languino LR, Ordentlich P, Liu Q, Xu X, Lo A, Pure E, Zhang C, Loboda A, Sepulveda MA, Snyder LA, Gabrilovich DI (2017) Cancer-associated fibroblasts neutralize the anti-tumor effect of CSF1 receptor blockade by inducing PMN-MDSC infiltration of tumors. Cancer Cell 32(5):654–668 e655. https://doi.org/10.1016/j.ccell.2017.10.005
McKane A, Sima C, Ramanathan RK, Jameson G, Mast C, White E, Fleck S, Downhour M, Von Hoff DD, Weiss GJ (2013) Determinants of patient screen failures in phase 1 clinical trials. Investig New Drugs 31(3):774–779. https://doi.org/10.1007/s10637-012-9894-7
Acknowledgements
We extend our gratitude to the patients and their families and caregivers for participating in this trial. Medical writing support was provided by Karan Sharma from Eli Lilly Services India Pvt. Ltd.
Funding
This trial was funded by Eli Lilly and Company.
Author information
Authors and Affiliations
Contributions
D. Yu, SC. Chapman, and AM. Szpurka contributed to study concept and design. D. Yu developed methodology. R. Wesolowski, SJ. Klempner, A. Dowlati, RH. Donald, and H. Omid contributed to patient and data acquisition. D. Yu, M. Carlsen, SC. Chapman, AM. Szpurka, R. Wesolowski, SJ. Klempner, A. Dowlati, RH. Donald, and H. Omid contributed to analysis and interpretation of data. All the authors contributed in writing, review, and revision of the manuscript.
Corresponding author
Ethics declarations
Ethics approval
The study was performed in accordance with the International Conference on Harmonization guidelines and Declaration of Helsinki 1964 and its amendments. The Institutional review board of each study site approved the study protocol, information brochure and the informed consent form before study initiation.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent for publication
Patients provided informed consent to regarding publishing their data.
Conflict of interest
A. Dowlati reports other from Glaxo Smith Kline, non-financial support from Glaxo Smith Kline; grants from EMD Serono, Tesaro, Roche, Vertex, Regeneron; grants and personal fees from Abbvie, Astra Zeneca, Millenium, Seattle Genetics; grants from Eli Lilly, Takeda, Ipsen, United Therapeutics, Mirati, Bristol Myers Squibb, Incuron; personal fees from Ariad, grants from Bayer.
RD. Harvey has nothing to disclose.
R. Carvajal reports personal fees and other from BMS, Incyte, Immunocore, Merck Roche/Genentech; personal fees from Castle Biosciences, Compugen, Foundation Medicine, I-Mab, PureTech, Sanofi Genzyme, Sorrento Therapeutics, Aura Biosciences, Chimeron, Rgenix; other from Amgen, Novartis, Pfizer, AstraZeneca, Bellicum, Plexxikon, Mirati, Macrogenics, Corvus, Bayer, Eli Lilly, Astellis, outside the submitted work.
O. Hamid received personal fees from Aduro, Akeso, Amgen, Array, Beigene, BMS, Genentech, GSK, Immunocore, Incyte, Janssen, Merck, Nextcure, Novartis, Sanofi Regeneron, Seattle Genetics, Tempus, Zelluna, Array Pfizer, BMS, Novartis, Sanofi Regeneron; other from Arcus, Aduro, Akeso, Amgen, Array, BMS, CytomX, Exelixis, Genentech, GSK, Immunocore, Incyte, Iovance, Merck, Moderna, Merck Serono, Nextcure, Novartis, Sanofi Regeneron, Seattle Genetics, Torque, Zelluna.
SJ. Klempner received personal fees from Eli Lilly, Merck, Bristol Myers Squibb, Foundation Medicine, Inc., Pieris; Stock/equity from Turning Point Therapeutics.
JSW. Kauh is a former employee of Eli Lilly and Company and presently working at Hutchison MediPharma Inc.
R. Wesolowski reports other (Lilly covered the cost of conducting this study at our institution).
AM. Szpurka, DA. Peterson, D. Yu, M. Carlsen, SC. Chapman, and T. Quinlan, are employees and stockholder at Eli Lilly and Company.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This manuscript is for a phase 1 clinical trial, therefore clinical trial related terms have been used.
Supplementary Information
ESM 1
(PDF 978 kb)
Rights and permissions
About this article
Cite this article
Dowlati, A., Harvey, R.D., Carvajal, R.D. et al. LY3022855, an anti–colony stimulating factor-1 receptor (CSF-1R) monoclonal antibody, in patients with advanced solid tumors refractory to standard therapy: phase 1 dose-escalation trial. Invest New Drugs 39, 1057–1071 (2021). https://doi.org/10.1007/s10637-021-01084-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-021-01084-8