Summary
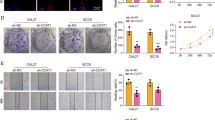
G9a, a histone methyltransferase, has been found to be upregulated in a range of tumor tissues, and contributes to tumor growth and metastasis. However, the impact of G9a inhibition as a potential therapeutic target in nasopharyngeal carcinoma (NPC) is unclear. In the present study we aimed to investigate the anti-proliferative effect of G9a inhibition in the NPC cell lines CNE1 and CNE2, and to further elucidate the molecular mechanisms underlying these effects. The expression of G9a in NPC tumor tissues was significantly higher than that in normal nasopharyngeal tissues. The pharmacological inhibition of G9a by BIX-01294 (BIX) inhibited proliferation and induced caspase-independent apoptosis in NPC cells in vitro. Treatment with BIX induced autophagosome accumulation, which exacerbated the cytotoxic activity of BIX in NPC cells. Mechanistic studies have found that BIX impairs autophagosomes by initiating autophagy in a Beclin-1-independent way, and impairs autophagic degradation by inhibiting lysosomal cathepsin D activation, leading to lysosomal dysfunction. BIX was able to suppress tumor growth, possibly by inhibiting autophagic flux; it might therefore constitute a promising candidate for NPC therapy.





Similar content being viewed by others
Data availability
All data needed to evaluate the conclusions in the paper are present in the paper.
References
Wei WI, Sham JS (2005) Nasopharyngeal carcinoma. Lancet 365(9476):2041–2054
Blanchard P, Lee A, Marguet S et al (2015) Chemotherapy and radiotherapy in nasopharyngeal carcinoma: an update of the MAC-NPC meta-analysis. Lancet Oncol 16(6):645–655
Hong JS, Tian J, Han QF, Ni QY (2015) Quality of life of nasopharyngeal cancer survivors in China. Curr Oncol 22(3):e142–e147
Shinkai Y, Tachibana M (2011) H3K9 methyltransferase G9a and the related molecule GLP. Genes Dev 25(8):781–788
Wang YF, Zhang J, Su Y et al (2017) G9a regulates breast cancer growth by modulating iron homeostasis through the repression of ferroxidase hephaestin. Nat Commun 8(1):274
Hua KT, Wang MY, Chen MW et al (2014) The H3K9 methyltransferase G9a is a marker of aggressive ovarian cancer that promotes peritoneal metastasis. Mol Cancer 13:189
Li KC, Hua KT, Lin YS et al (2014) Inhibition of G9a induces DUSP4-dependent autophagic cell death in head and neck squamous cell carcinoma. Mol Cancer 13:172
Li F, Zeng J, Gao Y et al (2015) G9a inhibition induces autophagic cell death via AMPK/mTOR pathway in bladder transitional cell carcinoma. PLoS One 10(9):e0138390
Kim Y, Kim YS, Kim DE et al (2013) BIX-01294 induces autophagy-associated cell death via EHMT2/G9a dysfunction and intracellular reactive oxygen species production. Autophagy 9(12):2126–2139
Rabinowitz JD, White E (2010) Autophagy and metabolism. Science 330(6009):1344–1348
Guo JY, Xia B, White E (2013) Autophagy-mediated tumor promotion. Cell 155(6):1216–1219
Wilde L, Tanson K, Curry J, Martinez-Outschoorn U (2018) Autophagy in cancer: a complex relationship. Biochem J 475(11):1939–1954
Degenhardt K, Mathew R, Beaudoin B et al (2006) Autophagy promotes tumor cell survival and restricts necrosis, inflammation, and tumorigenesis. Cancer Cell 10(1):51–64
Lei Y, Zhang D, Yu J, Dong H, Zhang J, Yang S (2017) Targeting autophagy in cancer stem cells as an anticancer therapy. Cancer Lett 393:33–39
Pan H, Wang Y, Na K et al (2019) Autophagic flux disruption contributes to Ganoderma lucidum polysaccharide-induced apoptosis in human colorectal cancer cells via MAPK/ERK activation. Cell Death Dis 10(6):456
Yang H, Gao Y, Fan X, Liu X, Peng L, Ci X (2019) Oridonin sensitizes cisplatin-induced apoptosis via AMPK/Akt/mTOR-dependent autophagosome accumulation in A549 cells. Front Oncol 9:769
Park SE, Yi HJ, Suh N et al (2016) Inhibition of EHMT2/G9a epigenetically increases the transcription of Beclin-1 via an increase in ROS and activation of NF-κB. Oncotarget 7(26):39796–39808
Ho JC, Abdullah LN, Pang QY et al (2017) Inhibition of the H3K9 methyltransferase G9A attenuates oncogenicity and activates the hypoxia signaling pathway. PLoS One 12(11):e0188051
Huang Y, Zou Y, Lin L, Ma X, Huang X (2017) Effect of BIX-01294 on proliferation, apoptosis and histone methylation of acute T lymphoblastic leukemia cells. Leuk Res 62:34–39
Woo SM, Seo SU, Min KJ, Kwon TK (2018) BIX-01294 sensitizes renal cancer Caki cells to TRAIL-induced apoptosis through downregulation of survivin expression and upregulation of DR5 expression. Cell Death Discov 4:29
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144(5):646–674
Cui J, Sun W, Hao X, Wei M, Su X, Zhang Y, Su L, Liu X (2015) EHMT2 inhibitor BIX-01294 induces apoptosis through PMAIP1-USP9X-MCL1 axis in human bladder cancer cells. Cancer Cell Int 15(1):4
Amaravadi RK, Kimmelman AC, Debnath J (2019) Targeting autophagy in cancer: recent advances and future directions. Cancer Discov 9(9):1167–1181
Carew JS, Kelly KR, Nawrocki ST (2012) Autophagy as a target for cancer therapy: new developments. Cancer Manag Res 4:357–365
Song Y, Li W, Peng X, Xie J, Li H, Tan G (2017) Inhibition of autophagy results in a reversal of taxol resistance in nasopharyngeal carcinoma by enhancing taxol-induced caspase-dependent apoptosis. Am J Transl Res 9(4):1934–1942
Zhu L, Li L, Zhang Q et al (2017) NOS1 S-nitrosylates PTEN and inhibits autophagy in nasopharyngeal carcinoma cells. Cell Death Discov 3:17011
Button RW, Roberts SL, Willis TL, Hanemann CO, Luo S (2017) Accumulation of autophagosomes confers cytotoxicity. J Biol Chem 292(33):13599–13614
Fu R, Deng Q, Zhang H et al (2018) A novel autophagy inhibitor berbamine blocks SNARE-mediated autophagosome-lysosome fusion through upregulation of BNIP3. Cell Death Dis 9(2):243
Luzio JP, Pryor PR, Bright NA (2007) Lysosomes: fusion and function. Nat Rev Mol Cell Biol 8(8):622–632
Dielschneider RF, Henson ES, Gibson SB (2017) Lysosomes as oxidative targets for cancer therapy. Oxid Med Cell Longev 2017:3749157
Towers CG, Thorburn A (2017) Targeting the lysosome for cancer therapy. Cancer Discov 7(11):1218–1220
Acknowledgements
The authors thank Editage (https://app.editage.cn/) for English language editing.
Funding
The Project was supported by grants from Chongqing Natural Science Foundation (Grant Serial Numbers: cstc2018jcyjAX0229) and Open Grants from the Key Laboratory of Electromagnetic Radiation Protection, Ministry of Education, China (No. 2017DCKF003).
Author information
Authors and Affiliations
Contributions
Qian Li performed cell lines studies and wrote the manuscript. Liuqian Wang, Di Ji, Xiaomin Bao, Guojing Tan and Xiaojun Liang collected tissue samples and performed the bioinformatics analysis. Ping Deng, Huifeng Pi, Yonghui Lu, Chunhai Chen, Mindi He and Lei Zhang provided critical experimental technology and helped with data analysis. Zhou Zhou and Zhengping Yu were responsible for revision of the manuscript. Anchun Deng initiated the study, oversaw the progress of the project, and offered guidance.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Ethics Committee of Xinqiao Hospital, Army Medical University and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
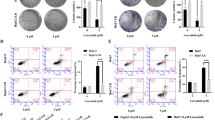
BIX-induced NPC cell viability inhibition does not occur by triggering apoptosis. (a) NPC cells were treated with different concentrations of BIX for 24 h. Apoptosis was then evaluated using flow cytometry, after double staining with annexin V and propidium iodide (PI). (b) The expression of cleaved caspase-3 and its substrate, cleaved PARP, was revealed using western blot analysis. A Jurkat cell total lysate was used as the positive control (PC) for the activation of caspase-3 and PARP. (c) NPC cells were treated with 10 μM BIX in the presence or absence of 20 μM Z-VAD for 24 h. Cell viability was determined using CCK-8 assay. Data are presented as mean ± SD. *P < 0.05, compared to the control. (PNG 4184 kb)
Rights and permissions
About this article
Cite this article
Li, Q., Wang, L., Ji, D. et al. BIX-01294, a G9a inhibitor, suppresses cell proliferation by inhibiting autophagic flux in nasopharyngeal carcinoma cells. Invest New Drugs 39, 686–696 (2021). https://doi.org/10.1007/s10637-020-01053-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-020-01053-7