Summary
Purpose
Part A of the open-label, phase I KEYNOTE-434 study evaluated the safety and tolerability of epacadostat, an indoleamine 2,3-dioxygenase-1 inhibitor, alone and in combination with pembrolizumab in Japanese patients with advanced solid tumors.
Methods
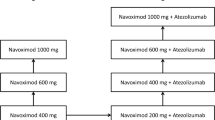
Japanese patients with refractory/recurrent metastatic or locally advanced tumors were enrolled. Cohort 1 received oral epacadostat 25 mg or 100 mg twice daily (BID) and subsequently received epacadostat in combination with intravenous pembrolizumab 200 mg every 3 weeks. Cohort 2 received epacadostat 25 mg or 100 mg BID with pembrolizumab 200 mg every 3 weeks. The primary objective was evaluation of safety and tolerability using a modified toxicity probability interval method. Secondary objectives were pharmacokinetic (PK) and pharmacodynamic profiles of epacadostat alone and in combination with pembrolizumab.
Results
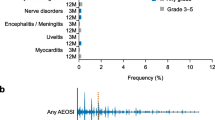
Six patients were enrolled in cohort 1 (epacadostat 25 mg, n = 3; epacadostat 100 mg, n = 3); none experienced dose-limiting toxicities (DLTs). Nine patients were enrolled in cohort 2 (epacadostat 25 mg and pembrolizumab, n = 3; epacadostat 100 mg and pembrolizumab, n = 6); one patient receiving epacadostat 100 mg and pembrolizumab experienced grade 4 rhabdomyolysis—a DLT. Grade 3 or 4 treatment-related adverse events occurred in two patients (13.3%). There were no treatment-related deaths. Pembrolizumab had no impact on epacadostat PK and vice versa. The PK profile of pembrolizumab in the current study was comparable with historical pembrolizumab PK data.
Conclusion
Epacadostat in combination with pembrolizumab was generally safe and well tolerated among Japanese patients with advanced solid tumors.
Clinical trial registration NCT02862457.




Similar content being viewed by others
References
Nixon NA, Blais N, Ernst S, Kollmannsberger C, Bebb G, Butler M, Smylie M, Verma S (2018) Current landscape of immunotherapy in the treatment of solid tumours, with future opportunities and challenges. Curr Oncol 25:e373–e384. https://doi.org/10.3747/co.25.3840
Topalian SL, Drake CG, Pardoll DM (2012) Targeting the PD-1/B7-H1(PD-L1) pathway to activate anti-tumor immunity. Curr Opin Immunol 24:207–212
Fukumura D, Kloepper J, Amoozgar Z, Duda DG, Jain RK (2018) Enhancing cancer immunotherapy using antiangiogenics: opportunities and challenges. Nat Rev Clin Oncol 15:325–340. https://doi.org/10.1038/nrclinonc.2018.29
Munn DH, Mellor AL (2013) Indoleamine 2,3 dioxygenase and metabolic control of immune responses. Trends Immunol 34:137–143. https://doi.org/10.1016/j.it.2012.10.001
Holmgaard RB, Zamarin D, Munn DH, Wolchok JD, Allison JP (2013) Indoleamine 2,3-dioxygenase is a critical resistance mechanism in antitumor T cell immunotherapy targeting CTLA-4. J Exp Med 210:1389–1402. https://doi.org/10.1084/jem.20130066
Spranger S, Koblish HK, Horton B, Scherle PA, Newton R, Gajewski TF (2014) Mechanism of tumor rejection with doublets of CTLA-4, PD-1/PD-L1, or IDO blockade involves restored IL-2 production and proliferation of CD8(+) T cells directly within the tumor microenvironment. J Immunother Cancer 2:3. https://doi.org/10.1186/2051-1426-2-3
Munn DH, Bronte V (2016) Immune suppressive mechanisms in the tumor microenvironment. Curr Opin Immunol 39:1–6. https://doi.org/10.1016/j.coi.2015.10.009
Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, Schadendorf D, Dummer R, Smylie M, Rutkowski P, Ferrucci PF, Hill A, Wagstaff J, Carlino MS, Haanen JB, Maio M, Marquez-Rodas I, McArthur GA, Ascierto PA, Long GV, Callahan MK, Postow MA, Grossmann K, Sznol M, Dreno B, Bastholt L, Yang A, Rollin LM, Horak C, Hodi FS, Wolchok JD (2015) Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med 373:23–34. https://doi.org/10.1056/NEJMoa1504030
Naidoo J, Page DB, Li BT, Connell LC, Schindler K, Lacouture ME, Postow MA, Wolchok JD (2015) Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann Oncol 26:2375–2391. https://doi.org/10.1093/annonc/mdv383
Hodi FS, Chesney J, Pavlick AC, Robert C, Grossmann KF, McDermott DF, Linette GP, Meyer N, Giguere JK, Agarwala SS, Shaheen M, Ernstoff MS, Minor DR, Salama AK, Taylor MH, Ott PA, Horak C, Gagnier P, Jiang J, Wolchok JD, Postow MA (2016) Combined nivolumab and ipilimumab versus ipilimumab alone in patients with advanced melanoma: 2-year overall survival outcomes in a multicentre, randomised, controlled, phase 2 trial. Lancet Oncol 17:1558–1568. https://doi.org/10.1016/S1470-2045(16)30366-7
Wolchok JD, Chiarion-Sileni V, Gonzalez R, Rutkowski P, Grob JJ, Cowey CL, Lao CD, Wagstaff J, Schadendorf D, Ferrucci PF, Smylie M, Dummer R, Hill A, Hogg D, Haanen J, Carlino MS, Bechter O, Maio M, Marquez-Rodas I, Guidoboni M, McArthur G, Lebbe C, Ascierto PA, Long GV, Cebon J, Sosman J, Postow MA, Callahan MK, Walker D, Rollin L, Bhore R, Hodi FS, Larkin J (2017) Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med 377:1345–1356. https://doi.org/10.1056/NEJMoa1709684
Hellmann MD, Rizvi NA, Goldman JW, Gettinger SN, Borghaei H, Brahmer JR, Ready NE, Gerber DE, Chow LQ, Juergens RA, Shepherd FA, Laurie SA, Geese WJ, Agrawal S, Young TC, Li X, Antonia SJ (2017) Nivolumab plus ipilimumab as first-line treatment for advanced non-small-cell lung cancer (CheckMate 012): results of an open-label, phase 1, multicohort study. Lancet Oncol 18:31–41. https://doi.org/10.1016/s1470-2045(16)30624-6
KEYTRUDA® (pembrolizumab) for injection, for intravenous use. Merck Sharp & Dohme Corp, Whitehouse Station, NJ; 4/2020
Yue EW, Sparks R, Polam P, Modi D, Douty B, Wayland B, Glass B, Takvorian A, Glenn J, Zhu W, Bower M, Liu X, Leffet L, Wang Q, Bowman KJ, Hansbury MJ, Wei M, Li Y, Wynn R, Burn TC, Koblish HK, Fridman JS, Emm T, Scherle PA, Metcalf B, Combs AP (2017) INCB24360 (epacadostat), a highly potent and selective indoleamine-2,3-dioxygenase 1 (IDO1) inhibitor for immuno-oncology. ACS Med Chem Lett 8:486–491. https://doi.org/10.1021/acsmedchemlett.6b00391
van Baren N, Van den Eynde BJ (2015) Tryptophan-degrading enzymes in tumoral immune resistance. Front Immunol 6:34. https://doi.org/10.3389/fimmu.2015.00034
Mitchell T. C, Hamid O, Smith D. C, Bauer T. M, Wasser J. S, Olszanski A. J, Luke J. J, Balmanoukian A. S, Schmidt E. V, Zhao Y, Gong X, Maleski J, Leopold L, Gajewski T. F (2018) Epacadostat plus pembrolizumab in patients with advanced solid tumors: phase I results from a multicenter, open-label phase I/II trial (ECHO-202/KEYNOTE-037). J Clin Oncol 36. doi:https://doi.org/10.1200/JCO.2018.78.9602
Ji Y, Wang SJ (2013) Modified toxicity probability interval design: a safer and more reliable method than the 3 + 3 design for practical phase I trials. J Clin Oncol 31:1785–1791. https://doi.org/10.1200/jco.2012.45.7903
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45:228–247. https://doi.org/10.1016/j.ejca.2008.10.026
National Cancer Institute. (2009) Common terminology criteria for adverse events (CTCAE). Version 4 May 28, 2009 https://ctepcancergov/protocolDevelopment/electronic_applications/ctchtm Accessed December 12, 2019
Nishino M, Giobbie-Hurder A, Hatabu H, Ramaiya NH, Hodi FS (2016) Incidence of programmed cell death 1 inhibitor-related pneumonitis in patients with advanced cancer: a systematic review and meta-analysis. JAMA Oncol 2:1607–1616. https://doi.org/10.1001/jamaoncol.2016.2453
Beatty GL, O'Dwyer PJ, Clark J, Shi JG, Bowman KJ, Scherle PA, Newton RC, Schaub R, Maleski J, Leopold L, Gajewski TF (2017) First-in-human phase I study of the oral inhibitor of indoleamine 2,3-dioxygenase-1 epacadostat (INCB024360) in patients with advanced solid malignancies. Clin Cancer Res 23:3269–3276. https://doi.org/10.1158/1078-0432.CCR-16-2272
European Medicines Agency (2016) Assessment Report: Keytruda. (Procedure No. EMEA/H/C/003820/II/0011)
Farkona S, Diamandis EP, Blasutig IM (2016) Cancer immunotherapy: the beginning of the end of cancer? BMC Med 14:73. https://doi.org/10.1186/s12916-016-0623-5
Alley EW, Lopez J, Santoro A, Morosky A, Saraf S, Piperdi B, van Brummelen E (2017) Clinical safety and activity of pembrolizumab in patients with malignant pleural mesothelioma (KEYNOTE-028): preliminary results from a non-randomised, open-label, phase 1b trial. Lancet Oncol 18:623–630. https://doi.org/10.1016/S1470-2045(17)30169-9
Shirai T, Sano T, Kamijo F, Saito N, Miyake T, Kodaira M, Katoh N, Nishie K, Okuyama R, Uhara H (2016) Acetylcholine receptor binding antibody-associated myasthenia gravis and rhabdomyolysis induced by nivolumab in a patient with melanoma. Jpn J Clin Oncol 46:86–88. https://doi.org/10.1093/jjco/hyv158
Liewluck T, Kao JC, Mauermann ML (2018) PD-1 inhibitor-associated myopathies: emerging immune-mediated myopathies. J Immunother 41:208–211. https://doi.org/10.1097/cji.0000000000000196
Namikawa K, Kiyohara Y, Takenouchi T, Uhara H, Uchi H, Yoshikawa S, Takatsuka S, Koga H, Wada N, Minami H, Hatsumichi M, Asada S, Namba Y, Yamazaki N (2018) Efficacy and safety of nivolumab in combination with ipilimumab in Japanese patients with advanced melanoma: an open-label, single-arm, multicentre phase II study. Eur J Cancer 105:114–126. https://doi.org/10.1016/j.ejca.2018.09.025
Long GV, Dummer R, Hamid O, Gajewski TF, Caglevic C, Dalle S, Arance A, Carlino MS, Grob JJ, Kim TM, Demidov L, Robert C, Larkin J, Anderson JR, Maleski J, Jones M, Diede SJ, Mitchell TC (2019) Epacadostat plus pembrolizumab versus placebo plus pembrolizumab in patients with unresectable or metastatic melanoma (ECHO-301/KEYNOTE-252): a phase 3, randomised, double-blind study. Lancet Oncol 20:1083–1097. https://doi.org/10.1016/s1470-2045(19)30274-8
Komiya T, Huang CH (2018) Updates in the clinical development of epacadostat and other indoleamine 2,3-dioxygenase 1 inhibitors (IDO1) for human cancers. Front Oncol 8:423. https://doi.org/10.3389/fonc.2018.00423
Acknowledgments
The authors thank all the nurses, data managers, patients, and their families for their outstanding dedicated patient care, careful data collection, participation, and time, respectively. The authors also thank Xiaohua Gong from Incyte for assistance with pharmacokinetic epacadostat assessments, and Kahori Sasahara, Yu Sakai, and Takashi Sawada from MSD K.K. for study management. Medical writing and/or editorial assistance was provided by Doyel Mitra, PhD, CMPP, and Brian Szente, PhD, of the ApotheCom pembrolizumab team (Yardley, PA). This assistance was funded by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, and Incyte Corporation, Wilmington, DE, USA.
Funding
Funding for this study was provided by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA and Incyte Corporation, Wilmington, DE, USA.
Author information
Authors and Affiliations
Contributions
Conception, design, or planning of the study: T.D., K.S., L.L., S.R.H., A.S.
Acquisition and analysis of data: T.D., Y.F., K.S., T.S., K.Y., N.M., I.O., T.K., Y.N., L.L., M.M., S.R.H., N.Yamamoto.
Interpretation of results: T.D., K.S., N.M., L.L., M.M., N.Yatsuzuka, A.S., N.Yamamoto.
Drafting of the manuscript: T.D., A.S.
Critically reviewing or revising the manuscript for important intellectual content: All authors.
Review of final version and agreement with content and decision to submit: All authors.
Corresponding author
Ethics declarations
Conflict of interest
T.D. received grants and personal fees from Eli Lilly, Kyowa Hakko Kirin, MSD, Daiichi Sankyo, Sumitomo Dainippon, Taiho, Novartis, Boehringer Ingelheim, Chugai Pharma, Bristol-Myers Squibb, AbbVie, personal fees from Amgen, Takeda, Bayer, Rakuten Medical, Ono Pharmaceutical, Astellas Pharma, Oncolys BioPharma, grants from Merck Serono, Janssen, Pfizer, Quintiles, and Eisai.
Y.F. received grants from MSD, AbbVie, AstraZeneca, Daiichi Sankyo, Eisai, Eli Lilly, Incyte, and Novartis for research funding; personal fees from AstraZeneca for advisory role and participation in speakers’ bureau, personal fees from Daiichi Sankyo for advisory role.
K.S. reports paid consulting or advisory roles for Astellas Pharma, Lilly, Bristol-Myers Squibb, Takeda, Pfizer, Ono Pharmaceutical, MSD, Taiho, Novartis, AbbVie, and GlaxoSmithKline; honoraria from Novartis, AbbVie, and Yakult; and research funding from Astellas, Lilly, Ono Pharmaceutical, Sumoitomo Dainippon, Daiichi Sankyo, Taiho, Chugai, MSD and Medi Science.
T.S. received research grant (Institute) from Novartis, Eli Lilly, Bristol-Myers Squibb, Daiichi Sankyo, Takeda Oncology, Eisai, AstraZeneca, AbbVie, Incyte, Astellas Pharma, Symbio Pharmaceuticals, 3D-Medicine, Five Prime, PharmaMar, and Chordia Therapeutics.
K.Y. received personal fees for advisory role and lecture from Eisai, personal fees for advisory role from Novartis, Chugai, and Ono Pharmaceutical, lecture fee from Taiho, personal fee for lecture from Pfizer.
N.M. received grants and personal fees from Janssen, MSD, Astra-Zeneca, grants from Roche, Lilly, Taiho, Bristol-Myers Squibb, and personal fees from Sanofi.
I.O., S.R.H., T.K., M.M., N.Yatsuzuka: have nothing to disclose.
L.L. reports employment at Incyte Corporation.
A.S. reports employment at Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA.
Y.N. received other for participation in speakers’ bureau from Pfizer, Taiho, Nippon Kayaku, Eli Lilly, AstraZeneca, Merck Serono, Bayer, Meiji Seika, Roche Diagnostics, Novartis, Taiho, Chugai Pharmaceutical, Eisai, and Fuji Film Toyama Chemistry; other for research funding from Roche Diagnostics.
N.Yamamoto received research grants from Chugai, Taiho, Eisai, Lilly, Quintiles, Astellas, Bristol-Myers Squibb, Novartis, Daiichi Sankyo, Pfizer, Boehringer Ingelheim, Kyowa-Hakko Kirin, Bayer, Ono Pharmaceutical, Takeda, Janssen Pharmaceutical, MSD, Merck, GSK, honoraria from Ono Pharmaceutical, Chugai, AstraZeneca, Pfizer, Lilly, Bristol-Myers Squibb, Sysmex, personal fees for consulting from Eisai, Otsuka, Takeda, Boehringer Ingelheim, and Cimic.
Ethical approval
The study protocol and all amendments were approved by the institutional review board or ethics committee at each center. The study was conducted in accordance with the protocol and its amendments, all relevant local and national regulations, the provisions of the Declaration of Helsinki, and Good Clinical Practice guidelines.
Informed consent
All participants provided written and informed consent before enrollment.
Availability of data and material
The data sharing policy of Merck Sharp & Dohme, including restrictions, is available at http://engagezone.msd.com/ds_documentation.php. Requests for access to the clinical study data can be submitted through the EngageZone site or via email to dataaccess@merck.com.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 484 kb)
Rights and permissions
About this article
Cite this article
Doi, T., Fujiwara, Y., Shitara, K. et al. The safety and tolerability of epacadostat alone and in combination with pembrolizumab in patients with advanced solid tumors: results from a first-in-Japanese phase I study (KEYNOTE-434). Invest New Drugs 39, 152–162 (2021). https://doi.org/10.1007/s10637-020-00942-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-020-00942-1