Summary
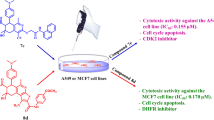
In a previous study we reported on the synthesis of 1,4-naphthoquinone-sulfides by thiolation of 1,4-naphthohydroquinones with primary aryl and alkyl thiols using laccase as catalyst. These compounds were synthesized as Vitamin K3 analogues. Vitamin K3 (VK3; 2-methyl-1,4-naphthoquinone; menadione) is known to have potent anticancer activity. This investigation reports on the anticancer activity of these VK3 analogues against TK10 renal, UACC62 melanoma, MCF7 breast, HeLa cervical, PC3 prostate and HepG2 liver cancer cell lines to evaluate their cytostatic effects. A 1,4-naphthohydroquinone derivative exhibited potent cytostatic effects (GI50 = 1.66–6.75 μM) which were better than that of etoposide and parthenolide against several of the cancer cell lines. This compound produces reactive oxygen species and disrupts the mitochondrial membrane potential in the MCF7 breast cancer cell line which is an indication that the cells undergo apoptosis. The 1,4-naphthoquinone sulfides also had potent cytostatic effects (GI50 = 2.82–9.79 μM) which were also better than that of etoposide, parthenolide and VK3 against several of the cancer cell lines. These compounds are generally more selective for cancer cells than for normal human lung fetal fibroblasts (WI-38). They also have moderate to weak cytostatic effects compared to etoposide, parthenolide and VK3 which have potent cytostatic effects against WI-38. One analogue induces apoptosis by activating caspases without arresting the cell cycle in the MCF7 breast cancer cell line. These results inspire further research for possible application in cancer chemotherapy.









Similar content being viewed by others
References
World Health organization (WHO) https://www.who.int/cancer/en/. Accessed 20 March 2019
WHO http://www.who.int/cancer/prevention/diagnosis-screening/breast-cancer/en/ Accessed 20 March 2019
WHO http://www.who.int/cancer/prevention/diagnosis-screening/cervical-cancer/en/. Accessed 20 March 2019
World Cancer Research Fund (WCRF) https://www.wcrf.org/dietandcancer/cancer-trends/prostate-cancer-statistics. Accessed 20 March 2019
WCRF https://www.wcrf.org/dietandcancer/cancer-trends/liver-cancer-statistics. Accessed 20 March 2019
WCRF https://www.wcrf.org/dietandcancer/cancer-trends/kidney-cancer-statistics. Accessed 20 March 2019
WHO http://www.who.int/uv/faq/skincancer/en/index1.html. Accessed 20 March 2019
Cranenburg EC, Schurgers LJ, Vermeer C (2007) Vitamin K: the coagulation vitamin that became omnipotent. Thromb Haemost 98:120–125
Lamson DW, Plaza SM (2003) The anticancer effects of vitamin K. Altern Med Rev 8:303–318
Parcell S (2002) Sulfur in human nutrition and applications in medicine. Altern MedRev 7:22–44
Wu X, Kassie F, Mersch-Sundermann V (2005) Induction of apoptosis in tumour cells by naturally occurring sulfur containing compounds. Mutat Res 589:81–102
Ariga T, Seki T (2006) Antithrombotic and anticancer effects of garlic-derived sulfur compounds: a review. Biofactors 26:93–103
Herman-Antosiewicz A, Powolny AA, Singh SV (2007) Molecular targets of cancer chemoprevention by garlic-derived organosulfides. Acta Pharmacol Sin 8:1355–1364
Shukla Y, Kalra N (2007) Cancer chemoprevention with garlic and its constituents. Cancer Lett 47:167–181
Tandon VK, Maurya HK, Mishra NN, Shukla PK (2009) Design, synthesis and biological evaluation of novel nitrogen and sulfur containing hetero-1,4-naphthoquinones as potent antifungal and antibacterial agents. Eur J Med Chem 44:3130–3137
Tandon VK, Maurya HK, Verma MK, Kumar R, Shukla PK (2010) 'On water' assisted synthesis and biological evaluation of nitrogen and sulfur containing hetero-1,4-naphthoquinones as potent antifungal and antibacterial agents. Eur J Med Chem 45:2418–2426
Huang G, Zhao HR, Meng QQ, Zhang QJ, Dong JY, Zhu BQ, Li SS (2018) Synthesis and biological evaluation of sulfur-containing shikonin oxime derivatives as potential antineoplastic agents. Eur J Med Chem 143:166–181
Kaufmann H, Earnshaw WC (2000) Induction of apoptosis by cancer chemotherapy. Exp Cell Res 256:42–49
Wellington KW, Kolesnikova NI (2012) A Laccase-catalysed one-pot synthesis of aminonaphthoquinones and their anticancer activity. Bioorg Med Chem 220:4472–4481
Wellington KW, Kolesnikova NI, Nyoka NBP, McGaw LJ (2018) Investigation of the antimicrobial and anticancer activity of aminonaphthoquinones. Drug Dev Res 80(1):138–146
Qwebani-Ogunleye T, Kolesnikova NI, Steenkamp P, De Koning CB, Brady D, Wellington KW (2017) A one-pot laccase-catalysed synthesis of coumestan derivatives and their anticancer activity. Bioorg Med Chem 25(3):1172–1182
Wellington KW, Qwebani-Ogunleye T, Kolesnikova NI, Brady D, de Koning CB (2013a) A one-pot laccase-catalysed synthesis of 5,6-dihydroxylated benzo[b]furans and catechol derivatives, and their anticancer activity. Arch Pharm (Weinheim) 346:266–277
Wellington KW, Gordon GER, Ndlovu LA, Kolesnikova NI, Steenkamp P (2013b) Laccase-catalysed C-S and C-C coupling for a one-pot synthesis of 1,4-Naphthoquinone sulfides and 1,4-Naphthoquinone sulfide dimers. ChemCatChem 5:1570–1577
Skehan P, Storeng R, Scudiero D, Monks A, McMahon J, Vistica D, Boyd MR (1990) New colorimetric cytotoxicity assay for anticancer drug screening. J Natl Cancer Inst 82:1107–1112
Perry SW, Norman JP, Barbieri J, Brown EB, Gelbard HA (2011) Mitochondrial membrane potential probes and the proton gradient: a practical usage guide. Biotechniques 50(2):98–115
Kaur M, Esau L (2015) Two step protocol for preparing adherent cells for high-throughput flow cytometry. Biotechniques 59(3):119–126
Yoon DS, Lee M-H, Cha DS (2018) Measurement of intracellular ROS in Caenorhabditis elegans using 2′, 7′-Dichlorodihydrofluorescein Diacetate. Bio–Protocol 8(6):e2774
Wu C-C, Li T-K, Farh L, Lin L-Y, Lin T-S, Yu Y-J, Yen T-J, Chiang C-W, Chan N-L (2011) Structural basis of type II topoisomerase inhibition by the anticancer drug Etoposide. Science 33:59–462
Carlisi D, D’anneo A, Angileri L, Lauricella M, Emanuele S, Santulli A, Vento R, Tesoriere G (2010) Parthenolide sensitizes hepatocellular carcinoma cells to TRAIL by inducing the expression of death receptors through inhibition of STAT3 activation. J Cell Physiol 226:1632–1641
Hayashi S, Koshiba K, Hatashita M, Sato T, Jujo Y, Suzuki R et al (2011) Thermosensitization and induction of apoptosis or cell-cycle arrest via the MAPK cascade by parthenolide, an NF-kB inhibitor, in human prostate cancer androgen-independent cell lines. Int J Mol Med 28:1033–1042
Zhang S, Ong CN, Shen HM (2004) Critical roles of intracellular thiols and calcium in parthenolide-induced apoptosis in human colorectal cancer cells. Cancer Lett 208:143–153
Akinboye ES, Bakare O (2011) Biological activities of emetine. Open Nat Product J 4:8–15
Janicke RU (2008) MCF-7 breast carcinoma cells do not express caspase-3. Breast Cancer Res Treat 117(1):219–221
Silverman RB (1992) The organic chemistry of drug design and drug action. Academic Press, New York
Lipinski CA (2004) Lead- and drug-like compounds: the rule-of-five revolution. Drug Discov Today Technol 1(4):337–341
Akiyoshi T, Matzno S, Sakai M, Okamura N, Matsuyama K (2009) The potential of vitamin K3 as an anticancer agent against breast cancer that acts via the mitochondria-related apoptotic pathway. Cancer Chemother Pharmacol 65:143–150
Nutter LM, Ngo EO, Fisher GR, Gutierrez PL (1992) DNA strand scission and free radical production in VK3-treated cells. Correlation with cytotoxicity and role of NADPH quinone acceptor oxidoreductase. J Biol Chem 267:2474–2479
Sakagami H, Satoh K, Hakeda Y, Kumegawa M (2000) Apoptosis-inducing activity of vitamin C and vitamin K. Cell Mol Biol 46(1):129–143
Yip KW, Reed JC (2008) Bcl-2 family proteins and cancer. Oncogene 27:6398–6406
Acknowledgements
We thank the CSIR (Thematic A grant) for financial support. The National Research Foundation Incentive Funding (No: 109163) and Claude Leon Foundation Merit Award (2016) to MK is also acknowledged.
Funding
This work was supported by the Biosciences unit, Council for Scientific and Industrial Research, Pretoria, South Africa.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Kevin W. Wellington declares that he has no conflict of interest. Natasha I. Kolesnikova declares that she has no conflict of interest. Vincent Hlatshwayo declares that he has no conflict of interest. Sourav Taru Saha declares that he has no conflict of interest. Mandeep Kaur declares that she has no conflict of interest. Lesetja. R. Motadi declares that he has no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
For this type of study, formal consent is not required.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 937 kb)
Rights and permissions
About this article
Cite this article
Wellington, K.W., Hlatshwayo, V., Kolesnikova, N.I. et al. Anticancer activities of vitamin K3 analogues. Invest New Drugs 38, 378–391 (2020). https://doi.org/10.1007/s10637-019-00855-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-019-00855-8