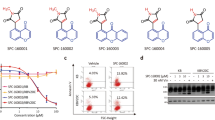
Summary
Microtubule targeting agents (MTAs) are extensively used in cancer treatment and many have achieved substantial clinical success. In recent years, targeting microtubules to inhibit cell division has become a widespread pharmaceutical approach for treatment of various cancer types. Nevertheless, the development of multidrug resistance (MDR) in cancer remains a major obstacle for successful application of these agents. Herein, we provided the evidence that CKT0353, α-branched α,β-unsaturated ketone, possesses the capacity to successfully evade the MDR phenotype as an MTA. CKT0353 induced G2/M phase arrest, delayed cell division via spindle assembly checkpoint activation, disrupted the mitotic spindle formation and depolymerized microtubules in human breast, cervix, and colorectal carcinoma cells. Molecular docking analysis revealed that CKT0353 binds at the nocodazole binding domain of β-tubulin. Furthermore, CKT0353 triggered apoptosis via caspase-dependent mechanism. In addition, P-glycoprotein overexpressing colorectal carcinoma cells showed higher sensitivity to this agent when compared to their sensitive counterpart, demonstrating the ability of CKT0353 to overcome this classic MDR mechanism involved in resistance to various MTAs. Taken together, these findings suggest that CKT0353 is an excellent candidate for further optimization as a therapeutic agent against tumors with MDR phenotype.







Similar content being viewed by others
References
Zhou J, Giannakakou P (2005) Targeting microtubules for cancer chemotherapy. Curr Med Chem Anticancer Agents 5(1):65–71
Jordan MA, Wilson L (2004) Microtubules as a target for anticancer drugs. Nat Rev Cancer 4(4):253–265. https://doi.org/10.1038/nrc1317
Downing KH (2000) Structural basis for the interaction of tubulin with proteins and drugs that affect microtubule dynamics. Annu Rev Cell Dev Biol 16:89–111. https://doi.org/10.1146/annurev.cellbio.16.1.89
Gottesman MM, Fojo T, Bates SE (2002) Multidrug resistance in cancer: role of ATP-dependent transporters. Nat Rev Cancer 2(1):48–58. https://doi.org/10.1038/nrc706
Riordan JR, Ling V (1985) Genetic and biochemical characterization of multidrug resistance. Pharmacol Ther 28(1):51–75
Sharom FJ (2008) ABC multidrug transporters: structure, function and role in chemoresistance. Pharmacogenomics 9(1):105–127. https://doi.org/10.2217/14622416.9.1.105
Ambudkar SV, Dey S, Hrycyna CA, Ramachandra M, Pastan I, Gottesman MM (1999) Biochemical, cellular, and pharmacological aspects of the multidrug transporter. Annu Rev Pharmacol Toxicol 39:361–398. https://doi.org/10.1146/annurev.pharmtox.39.1.361
Gonzalez-Garay ML, Chang L, Blade K, Menick DR, Cabral F (1999) A beta-tubulin leucine cluster involved in microtubule assembly and paclitaxel resistance. J Biol Chem 274(34):23875–23882
Kavallaris M, Tait AS, Walsh BJ, He L, Horwitz SB, Norris MD, Haber M (2001) Multiple microtubule alterations are associated with Vinca alkaloid resistance in human leukemia cells. Cancer Res 61(15):5803–5809
Giannakakou P, Gussio R, Nogales E, Downing KH, Zaharevitz D, Bollbuck B, Poy G, Sackett D, Nicolaou KC, Fojo T (2000) A common pharmacophore for epothilone and taxanes: molecular basis for drug resistance conferred by tubulin mutations in human cancer cells. Proc Natl Acad Sci U S A 97(6):2904–2909. https://doi.org/10.1073/pnas.040546297
Ganapathi RN, Ganapathi MK (2013) Mechanisms regulating resistance to inhibitors of topoisomerase II. Front Pharmacol 4:89. https://doi.org/10.3389/fphar.2013.00089
McGrogan BT, Gilmartin B, Carney DN, McCann A (2008) Taxanes, microtubules and chemoresistant breast cancer. Biochim Biophys Acta 1785(2):96–132. https://doi.org/10.1016/j.bbcan.2007.10.004
Goncalves A, Braguer D, Kamath K, Martello L, Briand C, Horwitz S, Wilson L, Jordan MA (2001) Resistance to Taxol in lung cancer cells associated with increased microtubule dynamics. Proc Natl Acad Sci U S A 98(20):11737–11742. https://doi.org/10.1073/pnas.191388598
Kavallaris M, Kuo DY, Burkhart CA, Regl DL, Norris MD, Haber M, Horwitz SB (1997) Taxol-resistant epithelial ovarian tumors are associated with altered expression of specific beta-tubulin isotypes. J Clin Invest 100(5):1282–1293. https://doi.org/10.1172/JCI119642
Podolski-Renic A, Bankovic J, Dinic J, Rios-Luci C, Fernandes MX, Ortega N, Kovacevic-Grujicic N, Martin VS, Padron JM, Pesic M (2017) DTA0100, dual topoisomerase II and microtubule inhibitor, evades paclitaxel resistance in P-glycoprotein overexpressing cancer cells. European Journal of Pharmaceutical Sciences: Eur J Pharm Sci 105:159–168. https://doi.org/10.1016/j.ejps.2017.05.011
Zhang CC, Yang JM, Bash-Babula J, White E, Murphy M, Levine AJ, Hait WN (1999) DNA damage increases sensitivity to vinca alkaloids and decreases sensitivity to taxanes through p53-dependent repression of microtubule-associated protein 4. Cancer Res 59(15):3663–3670
Zhang CC, Yang JM, White E, Murphy M, Levine A, Hait WN (1998) The role of MAP4 expression in the sensitivity to paclitaxel and resistance to vinca alkaloids in p53 mutant cells. Oncogene 16(12):1617–1624. https://doi.org/10.1038/sj.onc.1201658
Karpaviciene I, Cikotiene I, Padron JM (2013) Synthesis and antiproliferative activity of alpha-branched alpha,beta-unsaturated ketones. Eur J Med Chem 70:568–578. https://doi.org/10.1016/j.ejmech.2013.10.041
Podolski-Renic A, Andelkovic T, Bankovic J, Tanic N, Ruzdijic S, Pesic M (2011) The role of paclitaxel in the development and treatment of multidrug resistant cancer cell lines. Biomed Pharmacother 65(5):345–353. https://doi.org/10.1016/j.biopha.2011.04.015
Kepp O, Galluzzi L, Lipinski M, Yuan J, Kroemer G (2011) Cell death assays for drug discovery. Nat Rev Drug Discov 10(3):221–237. https://doi.org/10.1038/nrd3373
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Gascoigne KE, Taylor SS (2008) Cancer cells display profound intra- and interline variation following prolonged exposure to antimitotic drugs. Cancer Cell 14(2):111–122. https://doi.org/10.1016/j.ccr.2008.07.002
Rieder CL, Maiato H (2004) Stuck in division or passing through: what happens when cells cannot satisfy the spindle assembly checkpoint. Dev Cell 7(5):637–651. https://doi.org/10.1016/j.devcel.2004.09.002
Nakano H, Wang W, Hashizume C, Funasaka T, Sato H, Wong RW (2011) Unexpected role of nucleoporins in coordination of cell cycle progression. Cell Cycle 10(3):425–433. https://doi.org/10.4161/cc.10.3.14721
Fernandez-Martinez J, Rout MP (2009) Nuclear pore complex biogenesis. Curr Opin Cell Biol 21(4):603–612. https://doi.org/10.1016/j.ceb.2009.05.001
Michel L, Diaz-Rodriguez E, Narayan G, Hernando E, Murty VV, Benezra R (2004) Complete loss of the tumor suppressor MAD2 causes premature cyclin B degradation and mitotic failure in human somatic cells. Proc Natl Acad Sci U S A 101(13):4459–4464. https://doi.org/10.1073/pnas.0306069101
Decordier I, Cundari E, Kirsch-Volders M (2008) Mitotic checkpoints and the maintenance of the chromosome karyotype. Mutat Res 651(1–2):3–13. https://doi.org/10.1016/j.mrgentox.2007.10.020
Lampson MA, Kapoor TM (2005) The human mitotic checkpoint protein BubR1 regulates chromosome-spindle attachments. Nat Cell Biol 7(1):93–98. https://doi.org/10.1038/ncb1208
Sudakin V, Chan GK, Yen TJ (2001) Checkpoint inhibition of the APC/C in HeLa cells is mediated by a complex of BUBR1, BUB3, CDC20, and MAD2. J Cell Biol 154(5):925–936. https://doi.org/10.1083/jcb.200102093
Dalton WB, Yang VW (2009) Role of prolonged mitotic checkpoint activation in the formation and treatment of cancer. Future Oncol 5(9):1363–1370. https://doi.org/10.2217/fon.09.118
Israels ED, Israels LG (2000) The cell cycle. Oncologist 5(6):510–513
Harvey SL, Charlet A, Haas W, Gygi SP, Kellogg DR (2005) Cdk1-dependent regulation of the mitotic inhibitor Wee1. Cell 122(3):407–420. https://doi.org/10.1016/j.cell.2005.05.029
Blagosklonny MV (2007) Mitotic arrest and cell fate: why and how mitotic inhibition of transcription drives mutually exclusive events. Cell Cycle 6(1):70–74. https://doi.org/10.4161/cc.6.1.3682
Janicke RU, Sprengart ML, Wati MR, Porter AG (1998) Caspase-3 is required for DNA fragmentation and morphological changes associated with apoptosis. J Biol Chem 273(16):9357–9360
Walensky LD (2006) BCL-2 in the crosshairs: tipping the balance of life and death. Cell Death Differ 13(8):1339–1350. https://doi.org/10.1038/sj.cdd.4401992
Stanton RA, Gernert KM, Nettles JH, Aneja R (2011) Drugs that target dynamic microtubules: a new molecular perspective. Med Res Rev 31(3):443–481. https://doi.org/10.1002/med.20242
Jordan MA, Thrower D, Wilson L (1992) Effects of vinblastine, podophyllotoxin and nocodazole on mitotic spindles. Implications for the role of microtubule dynamics in mitosis. J Cell Sci 102(Pt 3):401–416
Xu K, Schwarz PM, Ludueña RF (2002) Interaction of nocodazole with tubulin isotypes. Drug Dev Res 55(2):91–96
Ghadimi BM, Sackett DL, Difilippantonio MJ, Schrock E, Neumann T, Jauho A, Auer G, Ried T (2000) Centrosome amplification and instability occurs exclusively in aneuploid, but not in diploid colorectal cancer cell lines, and correlates with numerical chromosomal aberrations. Genes Chromosom Cancer 27(2):183–190
Tsushimi T, Noshima S, Oga A, Esato K, Sasaki K (2001) DNA amplification and chromosomal translocations are accompanied by chromosomal instability: analysis of seven human colon cancer cell lines by comparative genomic hybridization and spectral karyotyping. Cancer Genet Cytogenet 126(1):34–38
Podolski-Renic A, Jadranin M, Stankovic T, Bankovic J, Stojkovic S, Chiourea M, Aljancic I, Vajs V, Tesevic V, Ruzdijic S, Gagos S, Tanic N, Pesic M (2013) Molecular and cytogenetic changes in multi-drug resistant cancer cells and their influence on new compounds testing. Cancer Chemother Pharmacol 72(3):683–697. https://doi.org/10.1007/s00280-013-2247-1
Chen Y, Simon SM (2000) In situ biochemical demonstration that P-glycoprotein is a drug efflux pump with broad specificity. J Cell Biol 148(5):863–870
Abdallah HM, Al-Abd AM, El-Dine RS, El-Halawany AM (2015) P-glycoprotein inhibitors of natural origin as potential tumor chemo-sensitizers: a review. J Adv Res 6(1):45–62. https://doi.org/10.1016/j.jare.2014.11.008
Kavallaris M, Burkhart CA, Horwitz SB (1999) Antisense oligonucleotides to class III beta-tubulin sensitize drug-resistant cells to Taxol. Br J Cancer 80(7):1020–1025. https://doi.org/10.1038/sj.bjc.6690507
Stengel C, Newman SP, Leese MP, Potter BV, Reed MJ, Purohit A (2010) Class III beta-tubulin expression and in vitro resistance to microtubule targeting agents. Br J Cancer 102(2):316–324. https://doi.org/10.1038/sj.bjc.6605489
Risinger AL, Jackson EM, Polin LA, Helms GL, LeBoeuf DA, Joe PA, Hopper-Borge E, Luduena RF, Kruh GD, Mooberry SL (2008) The taccalonolides: microtubule stabilizers that circumvent clinically relevant taxane resistance mechanisms. Cancer Res 68(21):8881–8888. https://doi.org/10.1158/0008-5472.CAN-08-2037
Acknowledgements
This work was performed within the framework of COST Action CA17104 STRATAGEM -” New diagnostic and therapeutic tools against multidrug resistant tumors”.
Funding
The work was supported by the Ministry of Education, Science and Technological Development of the Republic of Serbia (Grant No. III41031), the EU Research Potential (FP7-REGPOT- 2012-CT2012–31637-IMBRAIN), and the European Social Fund under the Global Grant measure (Grant No. VP1–3.1-ŠMM-07-K-01-002).We thank the Spanish Government for financial support through project PGC2018-094503-B-C22 (MCIU/AEI/FEDER, UE).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
Jelena Dinić declares that she has no conflict of interest. Carla Ríos-Luci declares that she has no conflict of interest. Ieva Karpaviciene declares that she has no conflict of interest. Inga Cikotiene declares that she has no conflict of interest. Miguel X. Fernandes declares that he has no conflict of interest. Milica Pešić declares that she has no conflict of interest. José M. Padrón declares that he has no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
For this type of study, formal consent is not required.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 691 kb)
Rights and permissions
About this article
Cite this article
Dinić, J., Ríos-Luci, C., Karpaviciene, I. et al. CKT0353, a novel microtubule targeting agent, overcomes paclitaxel induced resistance in cancer cells. Invest New Drugs 38, 584–598 (2020). https://doi.org/10.1007/s10637-019-00803-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-019-00803-6