Summary
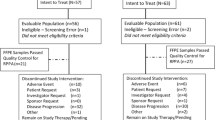
Background Imatinib mesylate is a potent inhibitor of the Abl, KIT and platelet derived growth factor (PDGF) receptor tyrosine kinases. Preclinical data suggest that combining imatinib mesylate with anti-estrogen therapy may be synergistic in hormone receptor-positive breast cancer. We report results of the first phase II trial evaluating the efficacy of the novel combination of imatinib mesylate and letrozole in the treatment of postmenopausal women with metastatic breast cancer. Patients and Methods 45 postmenopausal women with hormone receptor-positive metastatic breast cancer whose tumors demonstrated c-kit and/or PDGFR-β positivity were treated with imatinib mesylate 400 mg PO twice daily and letrozole 2.5 mg PO once daily until disease progression or unacceptable toxicity. Results There were no complete responses and five partial responses for an objective response rate of 11%. An additional 16 patients had stable disease lasting at least 24 weeks for a clinical benefit rate of 46.7%. The median progression-free and overall survival was 8.7 months (95% confidence interval: 3.8–11.4 months) and 44.3 months (95% confidence interval: 34.0–55.3 months), respectively. The most common grade 3 or higher treatment related adverse events were fatigue and diarrhea, occurring in 9 (20%) and 7 patients (16%), respectively. Conclusion The combination of imatinib mesylate and letrozole is well tolerated but appears to have limited efficacy in the treatment of hormone receptor-positive metastatic breast cancer.


Similar content being viewed by others
References
Mouridsen H, Gershanovich M, Sun Y, Perez-Carrion R, Boni C, Monnier A, Apffelstaedt J, Smith R, Sleeboom HP, Janicke F, Pluzanska A, Dank M, Becquart D, Bapsy PP, Salminen E, Snyder R, Lassus M, Verbeek JA, Staffler B, Chaudri-Ross HA, Dugan M (2001) Superior efficacy of letrozole versus tamoxifen as first-line therapy for postmenopausal women with advanced breast cancer: results of a phase III study of the international Letrozole breast Cancer group. J Clin Oncol 19(10):2596–2606. https://doi.org/10.1200/JCO.2001.19.10.2596
Knowlden JM, Hutcheson IR, Jones HE, Madden T, Gee JM, Harper ME, Barrow D, Wakeling AE, Nicholson RI (2003) Elevated levels of epidermal growth factor receptor/c-erbB2 heterodimers mediate an autocrine growth regulatory pathway in tamoxifen-resistant MCF-7 cells. Endocrinology 144(3):1032–1044. https://doi.org/10.1210/en.2002-220620
Kurokawa H, Lenferink AE, Simpson JF, Pisacane PI, Sliwkowski MX, Forbes JT, Arteaga CL (2000) Inhibition of HER2/neu (erbB-2) and mitogen-activated protein kinases enhances tamoxifen action against HER2-overexpressing, tamoxifen-resistant breast cancer cells. Cancer Res 60(20):5887–5894
Martin LA, Farmer I, Johnston SR, Ali S, Marshall C, Dowsett M (2003) Enhanced estrogen receptor (ER) alpha, ERBB2, and MAPK signal transduction pathways operate during the adaptation of MCF-7 cells to long term estrogen deprivation. J Biol Chem 278(33):30458–30468. https://doi.org/10.1074/jbc.M305226200
Mauro MJ, O'Dwyer M, Heinrich MC, Druker BJ (2002) STI571: a paradigm of new agents for cancer therapeutics. J Clin Oncol 20(1):325–334. https://doi.org/10.1200/JCO.2002.20.1.325
Heinrich MC, Griffith DJ, Druker BJ, Wait CL, Ott KA, Zigler AJ (2000) Inhibition of c-kit receptor tyrosine kinase activity by STI 571, a selective tyrosine kinase inhibitor. Blood 96(3):925–932
Franke TF, Yang SI, Chan TO, Datta K, Kazlauskas A, Morrison DK, Kaplan DR, Tsichlis PN (1995) The protein kinase encoded by the Akt proto-oncogene is a target of the PDGF-activated phosphatidylinositol 3-kinase. Cell 81(5):727–736
Jahn T, Seipel P, Urschel S, Peschel C, Duyster J (2002) Role for the adaptor protein Grb10 in the activation of Akt. Mol Cell Biol 22(4):979–991
Gesbert F, Sellers WR, Signoretti S, Loda M, Griffin JD (2000) BCR/ABL regulates expression of the cyclin-dependent kinase inhibitor p27Kip1 through the phosphatidylinositol 3-kinase/AKT pathway. J Biol Chem 275(50):39223–39230. https://doi.org/10.1074/jbc.M007291200
Chui X, Egami H, Yamashita J, Kurizaki T, Ohmachi H, Yamamoto S, Ogawa M (1996) Immunohistochemical expression of the c-kit proto-oncogene product in human malignant and non-malignant breast tissues. Br J Cancer 73(10):1233–1236
DiPaola RS, Kuczynski WI, Onodera K, Ratajczak MZ, Hijiya N, Moore J, Gewirtz AM (1997) Evidence for a functional kit receptor in melanoma, breast, and lung carcinoma cells. Cancer Gene Ther 4(3):176–182
Lammie A, Drobnjak M, Gerald W, Saad A, Cote R, Cordon-Cardo C (1994) Expression of c-kit and kit ligand proteins in normal human tissues. J Histochem Cytochem 42(11):1417–1425. https://doi.org/10.1177/42.11.7523489
Tsuura Y, Hiraki H, Watanabe K, Igarashi S, Shimamura K, Fukuda T, Suzuki T, Seito T (1994) Preferential localization of c-kit product in tissue mast cells, basal cells of skin, epithelial cells of breast, small cell lung carcinoma and seminoma/dysgerminoma in human: immunohistochemical study on formalin-fixed, paraffin-embedded tissues. Virchows Arch 424(2):135–141
Bhardwaj B, Klassen J, Cossette N, Sterns E, Tuck A, Deeley R, Sengupta S, Elliott B (1996) Localization of platelet-derived growth factor beta receptor expression in the periepithelial stroma of human breast carcinoma. Clin Cancer Res 2(4):773–782
Lev DC, Kim SJ, Onn A, Stone V, Nam DH, Yazici S, Fidler IJ, Price JE (2005) Inhibition of platelet-derived growth factor receptor signaling restricts the growth of human breast cancer in the bone of nude mice. Clin Cancer Res 11(1):306–314
Malavaki CJ, Roussidis AE, Gialeli C, Kletsas D, Tsegenidis T, Theocharis AD, Tzanakakis GN, Karamanos NK (2013) Imatinib as a key inhibitor of the platelet-derived growth factor receptor mediated expression of cell surface heparan sulfate proteoglycans and functional properties of breast cancer cells. FEBS J 280(10):2477–2489. https://doi.org/10.1111/febs.12163
Weigel MT, Meinhold-Heerlein I, Bauerschlag DO, Schem C, Bauer M, Jonat W, Maass N, Mundhenke C (2009) Combination of imatinib and vinorelbine enhances cell growth inhibition in breast cancer cells via PDGFR beta signalling. Cancer Lett 273(1):70–79. https://doi.org/10.1016/j.canlet.2008.07.040
Hines SJ, Litz JS, Krystal GW (1999) Coexpression of c-kit and stem cell factor in breast cancer results in enhanced sensitivity to members of the EGF family of growth factors. Breast Cancer Res Treat 58(1):1–10
Bagheri-Yarmand R, Kourbali Y, Morere JF, Jozefonvicz J, Crepin M (1998) Inhibition of MCF-7ras tumor growth by carboxymethyl benzylamide dextran: blockage of the paracrine effect and receptor binding of transforming growth factor beta1 and platelet-derived growth factor-BB. Cell Growth Differ 9(6):497–504
Roussidis AE, Mitropoulou TN, Theocharis AD, Kiamouris C, Papadopoulos S, Kletsas D, Karamanos NK (2004) STI571 as a potent inhibitor of growth and invasiveness of human epithelial breast cancer cells. Anticancer Res 24(3a):1445–1447
Pietras K, Ostman A, Sjoquist M, Buchdunger E, Reed RK, Heldin CH, Rubin K (2001) Inhibition of platelet-derived growth factor receptors reduces interstitial hypertension and increases transcapillary transport in tumors. Cancer Res 61(7):2929–2934
Pietras K, Rubin K, Sjoblom T, Buchdunger E, Sjoquist M, Heldin CH, Ostman A (2002) Inhibition of PDGF receptor signaling in tumor stroma enhances antitumor effect of chemotherapy. Cancer Res 62(19):5476–5484
Modi S, Seidman AD, Dickler M, Moasser M, D'Andrea G, Moynahan ME, Menell J, Panageas KS, Tan LK, Norton L, Hudis CA (2005) A phase II trial of imatinib mesylate monotherapy in patients with metastatic breast cancer. Breast Cancer Res Treat 90(2):157–163. https://doi.org/10.1007/s10549-004-3974-0
Cristofanilli M, Morandi P, Krishnamurthy S, Reuben JM, Lee BN, Francis D, Booser DJ, Green MC, Arun BK, Pusztai L, Lopez A, Islam R, Valero V, Hortobagyi GN (2008) Imatinib mesylate (Gleevec) in advanced breast cancer-expressing C-kit or PDGFR-beta: clinical activity and biological correlations. Ann Oncol 19(10):1713–1719. https://doi.org/10.1093/annonc/mdn352
Chew HK, Barlow WE, Albain K, Lew D, Gown A, Hayes DF, Gralow J, Hortobagyi GN, Livingston R (2008) A phase II study of imatinib mesylate and capecitabine in metastatic breast cancer: southwest oncology group study 0338. Clin Breast Cancer 8(6):511–515. https://doi.org/10.3816/CBC.2008.n.062
Mouridsen H, Gershanovich M, Sun Y, Perez-Carrion R, Boni C, Monnier A, Apffelstaedt J, Smith R, Sleeboom HP, Jaenicke F, Pluzanska A, Dank M, Becquart D, Bapsy PP, Salminen E, Snyder R, Chaudri-Ross H, Lang R, Wyld P, Bhatnagar A (2003) Phase III study of letrozole versus tamoxifen as first-line therapy of advanced breast cancer in postmenopausal women: analysis of survival and update of efficacy from the international Letrozole breast Cancer group. J Clin Oncol 21(11):2101–2109. https://doi.org/10.1200/JCO.2003.04.194
Finn RS, Crown JP, Lang I, Boer K, Bondarenko IM, Kulyk SO, Ettl J, Patel R, Pinter T, Schmidt M, Shparyk Y, Thummala AR, Voytko NL, Fowst C, Huang X, Kim ST, Randolph S, Slamon DJ (2015) The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol 16(1):25–35. https://doi.org/10.1016/S1470-2045(14)71159-3
Finn RS, Martin M, Rugo HS, Jones S, Im SA, Gelmon K, Harbeck N, Lipatov ON, Walshe JM, Moulder S, Gauthier E, Lu DR, Randolph S, Dieras V, Slamon DJ (2016) Palbociclib and Letrozole in advanced breast Cancer. N Engl J Med 375(20):1925–1936. https://doi.org/10.1056/NEJMoa1607303
Paulsson J, Ryden L, Strell C, Frings O, Tobin NP, Fornander T, Bergh J, Landberg G, Stal O, Ostman A (2017) High expression of stromal PDGFRbeta is associated with reduced benefit of tamoxifen in breast cancer. J Pathol Clin Res 3(1):38–43. https://doi.org/10.1002/cjp2.56
Acknowledgements
This work was supported in part by the National Institutes of Health/National Cancer Institute (NCI P30 CA016672). Dr. Giordano is supported by CPRIT RP160674 and Komen SAC150061. Dr. Yam is supported by the Allison and Brian Grove Endowed Fellowship for Breast Medical Oncology, the Susan Papizan Dolan Fellowship in Breast Oncology, and the 2018 Gianni Bonadonna Breast Cancer Research Fellowship. Any opinions, findings, and conclusions expressed in this material are those of the author(s) and do not necessarily reflect those of the sponsors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no relevant potential conflicts of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Yam, C., Murthy, R.K., Rauch, G.M. et al. A phase II study of imatinib mesylate and letrozole in patients with hormone receptor-positive metastatic breast cancer expressing c-kit or PDGFR-β. Invest New Drugs 36, 1103–1109 (2018). https://doi.org/10.1007/s10637-018-0672-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-018-0672-z