Summary
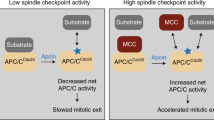
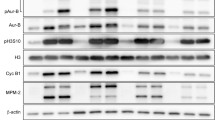
To ensure proper chromosome segregation, mitosis is tightly regulated by the spindle assembly checkpoint (SAC). Low concentrations of microtubule-stabilizing agents can induce aneuploid populations of cells in the absence of G2/M block, suggesting pertubation of the spindle checkpoint. We investigated the effects of peloruside A, a microtubule-stabilizing agent, on expression levels of several key cell cycle proteins, MAD2, BUBR1, p55CDC and cyclin B1. Synchronized 1A9 ovarian carcinoma cells were allowed to progress through the cell cycle in the presence or absence of peloruside A. Co-immunoprecipitation and Western blotting were used to probe the cell cycle kinetics of MAD2 and BUBR1 dissociation from p55CDC. Using confocal microscopy, we investigated whether premature dissociation of MAD2 and BUBR1 at low (40 nM) but not high (100 nM) concentrations of peloruside A was caused by defects in the attachment of chromosomes to the mitotic spindle. An increased frequency of polar chromosomes was observed at low concentrations of peloruside A, suggesting that an increased frequency of pseudo-metaphase cells, which are not detected by the spindle assembly checkpoint, may be underlying the induction of aneuploidy.







Similar content being viewed by others
Abbreviations
- APC/C:
-
Anaphase promoting complex/cyclosome
- BUBR1:
-
Budding uninhibited by benzimidazoles related 1
- CENP-E:
-
Centromere-associated protein-E
- CDK1:
-
Cyclin dependent kinase 1
- MAD2:
-
Mitotic arrest deficient 2
- MDA:
-
Microtubule-destabilizing agent
- MSA:
-
Microtubule-stabilizing agent
- MTA:
-
Microtubule-targeting agent
- PELA:
-
Peloruside A
- PTX:
-
Paclitaxel
- SAC:
-
Spindle assembly checkpoint
References
Jordan MA, Wilson L (2004) Microtubules as a target for anticancer drugs. Nat Rev Cancer 4:253–265
Dumontet C, Jordan MA (2010) Microtubule-binding agents: a dynamic field of cancer therapeutics. Nat Rev Drug Discov 9:790–803
Rowinsky EK, Eisenhauer EA, Chaudhry V, Arbuck SG, Donehower RC (1993) Clinical toxicities encountered with paclitaxel (Taxol). Semin Oncol 20:1–15
Gottesman MM, Fojo T, Bates SE (2002) Multidrug resistance in cancer: role of ATP–dependent transporters. Nat Rev Cancer 2:48–58
West LM, Northcote PT, Battershill CN (2000) Peloruside A: a potent cytotoxic macrolide isolated from the New Zealand marine sponge Mycale sp. J Organomet Chem 65:445–449
Hood KA, West LM, Rouwé B, Northcote PT, Berridge MV, Wakefield SJ, Miller JH (2002) Peloruside A, a novel antimitotic agent with paclitaxel-like microtubule stabilizing activity. Cancer Res 62:3356–3360
Gaitanos TN, Buey RM, Díaz F, Northcote PT, Teesdale-Spittle P, Andreu JM, Miller JH (2004) Peloruside A does not bind to the taxoid site on β-tubulin and retains its activity in multidrug-resistant cell lines. Cancer Res 64:5063–5067
Pryor DE, O'Brate A, Bilcer G, Díaz JF, Wang Y, Wang Y, Kabaki M, Jung MK, Andreu JM, Ghosh AK, Giannakakou P, Hamel E (2002) The microtubule stabilizing agent laulimalide does not bind in the taxoid site, kills cells resistant to paclitaxel and epothilones, and may not require its epoxide moiety for activity. Biochemistry 41:9109–9115
Prota AE, Bargsten K, Zurwerra D, Field JJ, Díaz JF, Altmann K-H, Steinmetz MO (2013) Molecular mechanism of action of microtubule-stabilizng agents. Science 339:587–590
Prota AE, Bargsten K, Northcote PT, Marsh M, Altmann K-H, Miller JH, Díaz JF, Steinmetz MO (2014) Structural basis of microtubule stabilization by laulimalide and peloruside A. Angew Chem Int Ed 53:1621–1625
Musacchio A, Salmon ED (2007) The spindle-assembly checkpoint in space and time. Nat Rev Mol Cell Biol 8:379–393
Lara-Gonzalez P, Westhorpe FG, Taylor SS (2012) The spindle assembly checkpoint. Curr Biol 22:R966–R980
Foley EA, Kapoor TM (2013) Microtubule attachment and spindle assembly checkpoint signalling at the kinetochore. Nat Rev Mol Cell Biol 14:25–37
Bharadwaj R, Yu H (2004) The spindle checkpoint, aneuploidy, and cancer. Oncogene 23:2016–2027
Thompson SL, Bakhoum SF, Compton DA (2010) Mechanisms of chromosomal instability. Curr Biol 20:R285–R295
Bakhoum SF, Compton DA (2012) Chromosomal instability and cancer: a complex relationship with therapeutic potential. J Clin Invest 122:1138–1143
Zachariae W, Nasmyth K (1999) Whose end is destruction: cell division and the anaphase-promoting complex. Genes Dev 13:2039–2058
Peters JM (2006) The anaphase promoting complex/cyclosome: a machine designed to destroy. Nat Rev Mol Cell Biol 7:644–656
Kallio M, Weinstein J, Daum JR, Burke DJ, Gorbsky GJ (1998) Mammalian p55CDC mediates association of the spindle checkpoint protein Mad2 with the cyclosome/anaphase-promoting complex, and is involved in regulating anaphase onset and late mitotic events. J Cell Biol 141:1393–1406
Sudakin V, Chan GK, Yen TJ (2001) Checkpoint inhibition of the APC/C in HeLa cells is mediated by a complex of BUBR1, BUB3, CDC20, and MAD2. J Cell Biol 154:925–936
Elowe S (2011) Bub1 and BubR1: at the interface between chromosome attachment and the spindle checkpoint. Mol Cell Biol 31:3085–3093
Chen J-G, Horwitz SB (2002) Differential mitotic responses to microtubule-stabilizing and -destabilizing drugs. Cancer Res 62:1935–1938
Chen J-G, Yang C-PH, Cammer M, Horwitz SB (2003) Gene expression and mitotic exit induced by microtubule-stabilizing drugs. Cancer Res 63:7891–7899
Wilmes A, Rawson P, Peng L, McLauchlan D, Northcote PT, Jordan TW, Miller JH (2011) Effects of the microtubule stabilizing agent peloruside A on the proteome of HL-60 cells. Investig New Drugs 29:544–553
Ikui AE, Yang CP, Matsumoto T, Horwitz SB (2005) Low concentrations of taxol cause mitotic delay followed by premature dissociation of p55CDC from Mad2 and BubR1 and abrogation of the spindle checkpoint, leading to aneuploidy. Cell Cycle 4:1385–1388
Wilmes A, Hanna R, Heathcott R, Northcote PT, Atkinson PH, Bellows DS, Miller JH (2012) Chemical genetic profiling of the microtubule-targeting agent peloruside A in budding yeast Saccharomyces cerevisiae. Gene 497:140–146
Best HA, Matthews JH, Heathcott RW, Hanna R, Leahy DC, Coorey NVC, Bellows DS, Atkinson PH, Miller JH (2013) Laulimalide and peloruside A inhibit mitosis of Saccharomyces cerevisiae by preventing microtubule depolymerisation-dependent steps in chromosome separation and nuclear positioning. Mol BioSyst 9:2842–2852
Ikui AE, Furuya K, Yanagida M, Matsumoto T (2002) Control of localization of a spindle checkpoint protein, Mad2, in fission yeast. J Cell Sci 115:1603–1610
Cimini D, Howell B, Maddox P, Khodjakov A, Degrassi F, Salmon ED (2001) Merotelic kinetochore orientation is a major mechanism of aneuploidy in mitotic mammalian tissue cells. J Cell Biol 153:517–527
Weaver BA, Bonday ZQ, Putkey FR, Kops GJ, Silk AD, Cleveland DW (2003) Centromere-associated protein-E is essential for the mammalian mitotic checkpoint to prevent aneuploidy due to single chromosome loss. J Cell Biol 162:551–563
Singh AJ, Razzak M, Teesdale-Spittle P, Gaitanos TN, Wilmes A, Paterson I, Goodman JM, Miller JH, Northcote PT (2011) Structure-activity studies of the pelorusides: new congeners and semi-synthetic analogues. Org Biomol Chem 9:4456–4466
Zacharaki P, Stephanou G, Demopoulos NA (2013) Comparison of the aneugenic properties of nocodazole, paclitaxel and griseofulvin in vitro. Centrosome defects and alterations in protein expression profiles. J Appl Toxicol 33:869–879
Lee H-S, Lee NC, Grimes BR, Samoshkin A, Kononenko AV, Bansal R, Masumoto H, Earnshaw WC, Kouprinal N, Larionov V (2013) A new assay for measuring chromosome instability (CIN) and identification of drugs that elevate CIN in cancer cells. BMC Cancer 13:252–264
Kanakkanthara A, Wilmes A, O'Brate A, Escuin D, Chan A, Gjyrezi A, Crawford J, Rawson P, Kivell B, Northcote PT, Hamel E, Giannakakou P, Miller JH (2011) Peloruside- and laulimalide-resistant human ovarian carcinoma cells have βI-tubulin mutations and altered expression of βII- and βIII-tubulin isotypes. Mol Cancer Ther 10:1419–1429
Chang DC, Xu N, Luo KQ (2003) Degradation of cyclin B is required for the onset of anaphase in mammalian cells. J Biol Chem 278:37865–37873
Brito DA, Rieder CL (2006) Mitotic checkpoint slippage in humans occurs via cyclin B destruction in the presence of an active checkpoint. Curr Biol 16:1194–1200
Brito DA, Yang Z, Rieder CL (2008) Microtubules do not promote mitotic slippage when the spindle assembly checkpoint cannot be satisfied. J Cell Biol 182:623–629
Yang Z, Loncarek J, Khodjakov A, Rieder CL (2008) Extra centrosomes and/or chromosomes prolong mitosis in human cells. Nat Cell Biol 10:748–751
Taylor SS, Hussein D, Wang Y, Elderkin S, Morrow CJ (2001) Kinetochore localization and phosphorylation of the mitotic checkpoint components Bub1 and BubR1 are differentially regulated by spindle events in human cells. J Cell Sci 114:4385–4395
Elowe S, Hümmer S, Uldschmid A, Li X, Nigg EA (2007) Tension-sensitive Plk1 phosphorylation on BubR1 regulates the stability of kinetochore microtubule interactions. Genes Dev 21:2205–2219
De Antoni A, Pearson CG, Cimini D, Canman JC, Sala V, Nezi L, Mapelli M, Sironi L, Faretta M, Salmon ED, Musacchio A (2005) The Mad1/Mad2 complex as a template for Mad2 activation in the spindle assembly checkpoint. Curr Biol 15:214–225
Yu H (2006) Structural activation of Mad2 in the mitotic spindle checkpoint: the two-state Mad2 model versus the Mad2 template model. J Cell Biol 173:153–157
Chen RH (2002) BubR1 is essential for kinetochore localization of other spindle checkpoint proteins and its phosphorylation requires Mad1. J Cell Biol 158:487–496
Morrow CJ, Tighe A, Johnson VL, Scott MI, Ditchfield C, Taylor SS (2005) Bub1 and aurora B cooperate to maintain BubR1-mediated inhibition of APC/CCdc20. J Cell Sci 118:3639–3652
Cimini D, Moree B, Canman JC, Salmon ED (2003) Merotelic kinetochore orientation occurs frequently during early mitosis in mammalian tissue cells and error correction is achieved by two different mechanisms. J Cell Sci 116:4213–4225
Rieder CL, Cole RW, Khodjakov A, Sluder G (1995) The checkpoint delaying anaphase in response to chromosome monoorientation is mediated by an inhibitory signal produced by unattached kinetochores. J Cell Biol 130:941–948
Jordan MA, Toso RJ, Thrower D, Wilson L (1993) Mechanism of mitotic block and inhibition of cell proliferation by taxol at low concentrations. Proc Natl Acad Sci U S A 90:9552–9556
Barisic M, Sousa RS, Tripathy SK, Magiera MM, Zaytsev AV, Pereira AL, Janke C, Grishchuk EL, Maiato H (2015) Microtubule detyrosination guides chromosomes during mitosis. Science 348:799–803
Gundersen GG, Khawaja S, Bulinksi JC (1987) Postpolymerization detyrosination of α-tubulin: a mechanism for subcellular differentiation of microtubules. J Cell Biol 105:251–264
Janssen A, Kops GJ, Medema RH (2009) Elevating the frequency of chromosome mis-segregation as a strategy to kill tumor cells. Proc Natl Acad Sci U S A 106:19108–19113
Acknowledgments
This work was supported by grants from the Cancer Society of New Zealand (JHM), Wellington Medical Research Foundation (JHM, PTN), and Victoria University of Wellington (JHM, AC) and a PhD scholarship from the Genesis Oncology Trust of New Zealand (AC).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Peter Northcote and John Miller are named on a patent for use of peloruside A as an anticancer agent.
Rights and permissions
About this article
Cite this article
Chan, A., Singh, A.J., Northcote, P.T. et al. Peloruside A, a microtubule-stabilizing agent, induces aneuploidy in ovarian cancer cells. Invest New Drugs 34, 424–438 (2016). https://doi.org/10.1007/s10637-016-0355-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-016-0355-6