Summary
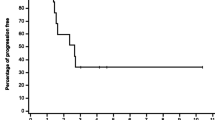
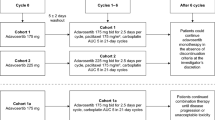
Purpose Preclinical evidence supports synergy between the vascular disrupting agent ombrabulin and various chemotherapy agents. Ombrabulin was combined with two standard taxane/platinum doublets in a phase I study to determine the recommended combination doses. Methods Ombrabulin (30-min infusion, day 1 every 3 weeks) was escalated from 15.5 to 35 mg/m2 with two chemotherapy doublets; OCD, 75 mg/m2 cisplatin (C), day 1 (cohort 1) or day 2 (cohort 2) with 60/75 mg/m2 docetaxel (D), day 2; and OCP, AUC5/6 carboplatin (C) and paclitaxel (P) 175 mg/m2 (cohort 3) or 200 mg/m2 (cohort 4), day 2. Safety, pharmacokinetics, and tumor response were evaluated. Results Sixty-nine patients were treated (32 OCD, 37 OCP). Four had DLTs in cycle 1, two in cohort 1 (grade 4 febrile neutropenia, grade 4 pulmonary embolism) and one each in cohorts 2 (grade 3 ALT elevation) and 4 (grade 3 peripheral ischemia). Ombrabulin escalation in cohorts 2, 3 and 4 was halted at the highest planned dose (35 mg/m2). Asthenia, nausea, paresthesia, alopecia, vomiting, and stomatitis were common, as was grade 3–4 neutropenia. Ombrabulin clearance was high with a short terminal half-life and a medium volume of distribution. Pharmacokinetic analysis showed no clinically relevant drug interactions between the taxane-platinum doublet and ombrabulin or its active metabolite RPR258063, however docetaxel and carboplatin pharmacokinetics were slightly altered. One complete and 15 partial responses (10 OCD, 5 OCP; median duration 5.5 and 4.4 months, respectively) were reported. Conclusions The addition of ombrabulin to standard doses of cisplatin/docetaxel or carboplatin/paclitaxel proved feasible with manageable overlapping toxicities but appears to have limited impact on the efficacy of these doublets. Recommended combination doses are 35 mg/m2 ombrabulin with 75 mg/m2 cisplatin/75 mg/m2 docetaxel or 200 mg/m2 paclitaxel/AUC6 carboplatin, every 3 weeks.

Similar content being viewed by others
References
McKeage MJ, Baguley BC (2010) Disrupting established tumor blood vessels: an emerging therapeutic strategy for cancer. Cancer 116:1859–1871
Nihei Y, Suzuki M, Okano A, Tsuji T, Akiyama Y, Tsuruo T et al (1999) Evaluation of antivascular and antimitotic effects of tubulin binding agents in solid tumor therapy. Jpn J Cancer Res 90:1387–1395
Hori K, Saito S, Kubota K (2002) A novel combretastatin A-4 derivative, AC7700, strongly stanches tumour blood flow and inhibits growth of tumours developing in various tissues and organs. Br J Cancer 86:1604–1614
Clemenson C, Jouannot E, Merino-Trigo A, Rubin-Carrez C, Deutsch E (2012) The vascular disrupting agent ombrabulin (AVE8062) enhances the efficacy of standard therapies in head and neck squamous cell carcinoma xenograft models. Invest New Drugs 31:273–284
Sessa C, Lorusso P, Tolcher A, Farace F, Lassau N, Delmonte A et al (2013) Phase I safety, pharmacokinetic and pharmacodynamic evaluation of the vascular disrupting agent ombrabulin (AVE8062) in patients with advanced solid tumors. Clin Cancer Res 19:4832–4842
Soria J, Sessa C, Perotti A, Massard C, Armand J, Lassaud N et al. (2008) A comprehensive study of translational research and safety exploration of the vascular disrupting agent (VDA) AVE8062 in combination with cisplatin administered every 3 weeks to patients with advanced solid tumors. Proceedings of the American Association for Cancer Research 99th Annual Meeting; Abstract LB-302.
Eskens F, Tresca P, Tosi D, Van Doorn L, Fontaine H, Van der Gaast A et al (2014) A phase I dose escalation and pharmacokinetic study of the vascular disrupting agent ombrabulin (AVE8062) combined with docetaxel in advanced solid tumors. Br J Cancer 110:2170–2177
Bruno R, Vivier N, Vergniol JC, De Phillips SL, Montay G, Sheiner LB (1996) A population pharmacokinetic model for docetaxel (taxotere): model building and validation. J Pharmacokinet Biopharm 24:153–172
Bruno R, Hille D, Riva A, Vivier N, ten Bokkel Huinnink WW, van Oosterom AT et al (1998) Population pharmacokinetics/pharmacodynamics of docetaxel in phase II studies in patients with cancer. J Clin Oncol 16:187–196
Harvey V, Mouridsen H, Semiglazov V, Jakobsen E, Voznyi E, Robinson BA et al (2006) Phase III trial comparing three doses of docetaxel for second-line treatment of advanced breast cancer. J Clin Oncol 24:4963–4970
Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A et al (2006) Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med 355:2542–2550
Lara PN Jr, Douillard JY, Nakagawa K, von Pawel J, McKeage MJ, Albert I et al (2011) Randomized phase III placebo-controlled trial of carboplatin and paclitaxel with or without the vascular disrupting agent vadimezan (ASA404) in advanced non-small-cell lung cancer. J Clin Oncol 29:2965–2971
Scagliotti GV, Vynnychenko I, Park K, Ichinose Y, Kubota K, Blackhall F et al (2012) International, randomized, placebo-controlled, double-blind phase III study of motesanib plus carboplatin/paclitaxel in patients with advanced nonsquamous non-small-cell lung cancer: MONET1. J Clin Oncol 30:2829–2836
du Bois A, Huober J, Stopfer P, Pfisterer J, Wimberger P, Loibl S et al (2010) A phase I open-label dose-escalation study of oral BIBF 1120 combined with standard paclitaxel and carboplatin in patients with advanced gynecological malignancies. Ann Oncol 21:370–375
Zweifel M, Jayson GC, Reed NS, Osborne R, Hassan B, Ledermann J et al (2011) Phase II trial of combretastatin A4 phosphate, carboplatin, and paclitaxel in patients with platinum-resistant ovarian cancer. Ann Oncol 22:2036–2041
Schiller JH, Harrington D, Belani CP, Langer C, Sandler A, Krook J et al (2002) Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med 346:92–98
Subbiah IM, Lenihan DJ, Tsimberidou AM (2011) Cardiovascular toxicity profiles of vascular-disrupting agents. Oncologist 16:1120–1130
Felici A, Loos WJ, Verweij J, Cirillo I, de Bruijn P, Nooter K et al (2006) A pharmacokinetic interaction study of docetaxel and cisplatin plus or minus 5-fluorouracil in the treatment of patients with recurrent or metastatic solid tumors. Cancer Chemother Pharmacol 58:673–680
Vergote I, Amant F, Oskay-Oezcelik G, Musib L, Michel AL, Darstein C et al (2009) Carboplatin and paclitaxel in combination with oral enzastaurin in advanced ovarian or primary peritoneal cancer: results from a safety lead-in study. Int J Gynecol Cancer 19:1505–1510
Oguri S, Sakakibara T, Mase H, Shimizu T, Ishikawa K, Kimura K et al (1988) Clinical pharmacokinetics of carboplatin. J Clin Pharmacol 28:208–215
Shea TC, Flaherty M, Elias A, Eder JP, Antman K, Begg C et al (1989) A phase I clinical and pharmacokinetic study of carboplatin and autologous bone marrow support. J Clin Oncol 7:651–661
Morinaga Y, Suga Y, Ehara S, Harada K, Nihei Y, Suzuki M (2003) Combination effect of AC-7700, a novel combretastatin A-4 derivative, and cisplatin against murine and human tumors in vivo. Cancer Sci 94:200–204
Rowinsky EK, Gilbert MR, McGuire WP, Noe DA, Grochow LB, Forastiere AA et al (1991) Sequences of taxol and cisplatin: a phase I and pharmacologic study. J Clin Oncol 9:1692–1703
Martinelli M, Bonezzi K, Riccardi E, Kuhn E, Frapolli R, Zucchetti M et al (2007) Sequence dependent antitumour efficacy of the vascular disrupting agent ZD6126 in combination with paclitaxel. Br J Cancer 97:888–894
Acknowledgments
We thank Florent Mazuir (Sanofi, France) for pharmacokinetics analyses, Essediq El-Yandouzi (Sanofi, France) for study management support, Jean-Christophe Quirot (Sanofi, France) for programming support, and Sarah MacKenzie (Medi-Axe, France, funded by Sanofi) for assistance writing the manuscript.
Funding
This study was supported by Sanofi.
Disclosure
The institutes of CS, LG, and JCS received a study grant from Sanofi. CO, BD and MH are employees of Sanofi. All other authors have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bahleda, R., Sessa, C., Del Conte, G. et al. Phase I clinical and pharmacokinetic study of ombrabulin (AVE8062) combined with cisplatin/docetaxel or carboplatin/paclitaxel in patients with advanced solid tumors. Invest New Drugs 32, 1188–1196 (2014). https://doi.org/10.1007/s10637-014-0119-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-014-0119-0