Abstract
Purpose
Alport syndrome comprises a heterogeneous group of inherited kidney diseases that are associated with ocular complications. In this study, we aimed to detail the clinical characteristics of a patient with X-linked Alport syndrome.
Methods
We performed next-generation sequencing (NGS) with hybridization capture to identify the disease-causing variant of Alport syndrome and a comprehensive ophthalmic examination, including full-field electroretinography (FF-ERG).
Results
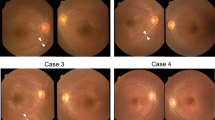
Genetic testing using NGS with hybridization capture revealed a novel hemizygous variant [c.51_52delGA (p.Trp20GlyfsTer19)] in exon 1 of COL4A5. The patient underwent cataract surgery in both eyes because of decreased visual acuity and photophobia. The best-corrected visual acuity improved from 0.9 and 0.7 in the right and left eyes, respectively, to 1.5 in both eyes. Anterior-segment optical coherence tomography (OCT) revealed anterior and posterior lenticonus. Fundus photographs showed central and peripheral fleck retinopathy. Wide-field fundus autofluorescence (AF) imaging showed mottled hyper- and hypo-AF in the peripheral retina, which was consistent with peripheral fleck retinopathy. Furthermore, OCT revealed thinning of the inner retinal layers, especially at the temporal macular, but the outer retinal layers were preserved. Ganglion cell analysis showed no progression for 5 years. FF-ERG was performed at 41 (phakia) and 46 (pseudophakia) years of age. The amplitudes of dark-adapted (DA) and light-adapted (LA) responses showed selective b-wave abnormalities. The b/a-wave ratios of DA 3.0 were 1.22 and 1.16 in the right and left eyes, respectively. The amplitudes of DA 3.0 oscillatory potentials (OP) were reduced. Five years later, the amplitudes of DA and LA responses revealed no remarkable changes, except for an OP wave of DA 3.0, which was substantially reduced.
Conclusions
Our findings revealed electroretinographic abnormalities in a patient with Alport syndrome, which predominantly indicated impairment of the inner retina. Notably, little short-term progression was observed.






Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article.
References
Colville DJ, Savige J (1997) Alport syndrome. A review of the ocular manifestations. Ophthalmic Genet 18:161–73
Savige J, Colville D (2009) Opinion: ocular features aid the diagnosis of Alport syndrome. Nat Rev Nephrol 5:356–360
Feingold J, Bois E, Chompret A, Broyer M, Gubler MC, Grünfeld JP (1985) Genetic heterogeneity of Alport syndrome. Kidney Int 27:672–677
Barker DF, Hostikka SL, Zhou J, Chow LT, Oliphant AR, Gerken SC, Gregory MC, Skolnick MH, Atkin CL, Tryggvason K (1990) Identification of mutations in the COL4A5 collagen gene in Alport syndrome. Science 248:1224–1227
Mochizuki T, Lemmink HH, Mariyama M, Antignac C, Gubler MC, Pirson Y, Verellen-Dumoulin C, Chan B, Schröder CH, Smeets HJ, Reeders ST (1994) Identification of mutations in the alpha 3(IV) and alpha 4(IV) collagen genes in autosomal recessive Alport syndrome. Nat Genet 8:77–81
Hudson BG, Reeders ST, Tryggvason K (1993) Type IV collagen: structure, gene organization, and role in human diseases. Molecular basis of Goodpasture and Alport syndromes and diffuse leiomyomatosis. J Biol Chem 268:26033–26036
Hudson BG, Tryggvason K, Sundaramoorthy M, Neilson EG (2003) Alport’s syndrome, Goodpasture’s syndrome, and type IV collagen. N Engl J Med 348:2543–2556
Kabosova A, Azar DT, Bannikov GA, Campbell KP, Durbeej M, Ghohestani RF, Jones JC, Kenney MC, Koch M, Ninomiya Y, Patton BL, Paulsson M, Sado Y, Sage EH, Sasaki T, Sorokin LM, Steiner-Champliaud MF, Sun TT, Sundarraj N, Timpl R, Virtanen I, Ljubimov AV (2007) Compositional differences between infant and adult human corneal basement membranes. Invest Ophthalmol Vis Sci 48:4989–4999
Savige J, Liu J, DeBuc DC, Handa JT, Hageman GS, Wang YY, Parkin JD, Vote B, Fassett R, Sarks S, Colville D (2010) Retinal basement membrane abnormalities and the retinopathy of Alport syndrome. Invest Ophthalmol Vis Sci 51:1621–1627
Rhys C, Snyers B, Pirson Y (1997) Recurrent corneal erosion associated with Alport's syndrome. Rapid communication. Kidney Int. 52:208–211
Ohkubo S, Takeda H, Higashide T, Ito M, Sakurai M, Shirao Y, Yanagida T, Oda Y, Sado Y (2003) Immunohistochemical and molecular genetic evidence for type IV collagen alpha5 chain abnormality in the anterior lenticonus associated with Alport syndrome. Arch Ophthalmol 121:846–850
Sonarkhan S, Ramappa M, Chaurasia S, Mulay K (2014) Bilateral anterior lenticonus in a case of Alport syndrome: a clinical and histopathological correlation after successful clear lens extraction. BMJ Case Rep. 2014
Wang Y, Sivakumar V, Mohammad M, Colville D, Storey H, Flinter F, Dagher H, Savige J (2014) Clinical and genetic features in autosomal recessive and X-linked Alport syndrome. Pediatr Nephrol 29:391–396
Shaw EA, Colville D, Wang YY, Zhang KW, Dagher H, Fassett R, Guymer R, Savige J (2007) Characterization of the peripheral retinopathy in X-linked and autosomal recessive Alport syndrome. Nephrol Dial Transplant 22:104–108
Mete UO, Karaaslan C, Ozbilgin MK, Polat S, Tap O, Kaya M (1996) Alport’s syndrome with bilateral macula hole. Acta Ophthalmol Scand 74:77–80
Usui T, Ichibe M, Hasegawa S, Miki A, Baba E, Tanimoto N, Abe H (2004) Symmetrical reduced retinal thickness in a patient with Alport syndrome. Retina 24:977–979
Gross O, Netzer KO, Lambrecht R, Seibold S, Weber M (2002) Meta-analysis of genotype-phenotype correlation in X-linked Alport syndrome: impact on clinical counselling. Nephrol Dial Transplant 17:1218–1227
Savige J, Sheth S, Leys A, Nicholson A, Mack HG, Colville D (2015) Ocular features in Alport syndrome: pathogenesis and clinical significance. Clin J Am Soc Nephrol 10:703–709
Robson AG, Frishman LJ, Grigg J, Hamilton R, Jeffrey BG, Kondo M, Li S, McCulloch DL (2022) ISCEV Standard for full-field clinical electroretinography (2022 update). Doc Ophthalmol 144:165–77
Mizobuchi K, Hayashi T, Matsuura T, Nakano T (2022) Clinical characterization of autosomal dominant retinitis pigmentosa with NRL mutation in a three-generation Japanese family. Doc Ophthalmol 144:227–235
Hayashi T, Hosono K, Kurata K, Katagiri S, Mizobuchi K, Ueno S, Kondo M, Nakano T, Hotta Y (2020) Coexistence of GNAT1 and ABCA4 variants associated with Nougaret-type congenital stationary night blindness and childhood-onset cone-rod dystrophy. Doc Ophthalmol 140:147–157
Ninomiya W, Mizobuchi K, Hayashi T, Okude S, Katagiri S, Kubo A, Masuhara N, Nakano T (2020) Electroretinographic abnormalities associated with pregabalin: a case report. Doc Ophthalmol 140:279–287
Kameya S, Fujinami K, Ueno S, Hayashi T, Kuniyoshi K, Ideta R, Kikuchi S, Kubota D, Yoshitake K, Katagiri S, Sakuramoto H, Kominami T, Terasaki H, Yang L, Fujinami-Yokokawa Y, Liu X, Arno G, Pontikos N, Miyake Y, Iwata T, Tsunoda K (2019) Phenotypical characteristics of POC1B-associated retinopathy in Japanese cohort: cone dystrophy with normal funduscopic appearance. Invest Ophthalmol Vis Sci 60:3432–3446
Kubota D, Matsumoto K, Hayashi M, Oishi N, Gocho K, Yamaki K, Kobayakawa S, Igarashi T, Takahashi H, Kameya S (2020) High-resolution photoreceptor imaging analysis of patients with autosomal dominant retinitis pigmentosa (adRP) caused by HK1 mutation. Ophthalmic Genet 41:629–638
Fujiki R, Ikeda M, Yoshida A, Akiko M, Yao Y, Nishimura M, Matsushita K, Ichikawa T, Tanaka T, Morisaki H, Morisaki T, Ohara O (2018) Assessing the accuracy of variant detection in cost-effective gene panel testing by next-generation sequencing. J Mol Diagn 20:572–582
Mizobuchi K, Hayashi T, Yoshitake K, Fujinami K, Tachibana T, Tsunoda K, Iwata T, Nakano T (2020) Novel homozygous CLN3 missense variant in isolated retinal dystrophy: a case report and electron microscopic findings. Mol Genet Genomic Med.e1308
Robinson JT, Thorvaldsdottir H, Winckler W, Guttman M, Lander ES, Getz G, Mesirov JP (2011) Integrative genomics viewer. Nat Biotechnol 29:24–26
Tan R, Colville D, Wang YY, Rigby L, Savige J (2010) Alport retinopathy results from “severe” COL4A5 mutations and predicts early renal failure. Clin J Am Soc Nephrol 5:34–38
Govan JA (1983) Ocular manifestations of Alport’s syndrome: a hereditary disorder of basement membranes? Br J Ophthalmol 67:493–503
Hess K, Pfau M, Wintergerst MWM, Loeffler KU, Holz FG, Herrmann P (2020) Phenotypic spectrum of the foveal configuration and foveal avascular zone in patients with alport syndrome. Invest Ophthalmol Vis Sci 61:5
Newman EA, Odette LL (1984) Model of electroretinogram b-wave generation: a test of the K+ hypothesis. J Neurophysiol 51:164–182
Tian N, Slaughter MM (1995) Correlation of dynamic responses in the ON bipolar neuron and the b-wave of the electroretinogram. Vision Res 35:1359–1364
Sieving PA, Murayama K, Naarendorp F (1994) Push-pull model of the primate photopic electroretinogram: a role for hyperpolarizing neurons in shaping the b-wave. Vis Neurosci 11:519–532
Gurevich L, Slaughter MM (1993) Comparison of the waveforms of the ON bipolar neuron and the b-wave of the electroretinogram. Vis Res 33:2431–2435
Acknowledgements
We thank the patients and their families for their participation in this study. We would also like to thank Prof. Sae Ochi and Ms. Ritsuko Nakayama for assisting in the genetic analysis.
Funding
This work was supported in part by JSPS KAKENHI grant no. 21K09756.
Author information
Authors and Affiliations
Contributions
All authors have contributed to the conception and design of this study. Material preparation, data collection, and analysis were performed by KM, TH, RO, and TN. The first draft of the manuscript was written by KM, and all authors commented on the previous versions of the manuscript. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Takaaki Hayashi received research funds from Alcon (Tokyo, Japan), Johnson & Johnson Vision, AMO (Tokyo, Japan), Daiichi Sankyo (Tokyo, Japan), Chugai (Tokyo, Japan), Mitsubishi Tanabe Pharma (Osaka, Japan), Senju (Osaka, Japan), Bayer (Osaka, Japan), Ritz Medical (Aichi, Japan), Uni-Hite (Kanagawa, Japan), Kuribara (Gunma, Japan), Kowa (Aichi, Japan), Kyowa Kirin (Tokyo, Japan), and Otsuka Pharma (Tokyo, Japan). The funders had no role in study design, data collection and analysis, manuscript preparation, or decision to publish.
Ethical approval
The study protocol was approved by the Institutional Review Board of the Jikei University School of Medicine (approval no. 24–231 6997). The protocol adhered to the tenets of the Declaration of Helsinki.
Statement of human rights
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Statement on the welfare of animals
This article does not contain any studies with animals performed by any of the authors.
Informed consent
Written informed consent was obtained from the patient for publication of this case report.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mizobuchi, K., Hayashi, T., Ohira, R. et al. Electroretinographic abnormalities in Alport syndrome with a novel COL4A5 truncated variant (p.Try20GlyfsTer19). Doc Ophthalmol 146, 281–291 (2023). https://doi.org/10.1007/s10633-023-09935-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10633-023-09935-w