Abstract
Purpose
To determine the association of the multifocal electroretinographic (mfERG) response amplitude with the volumes of the inner, postreceptor, and photoreceptor retinal layers in the region stimulated by each mfERG element.
Methods
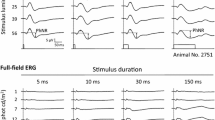
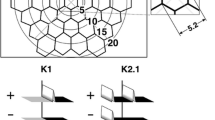
Sixteen healthy, young adult control subjects were studied. Each of the 103 hexagonal elements of the standard, scaled mfERG were aligned, where possible, with patches of retina imaged using optical coherence tomography. Stimuli falling on the fovea and on the optic nerve head were excluded. Linear mixed-effects modeling was then used to derive estimated coefficients (voltage/volume) for the mfERG response throughout the full 80 ms standard epoch. The resulting predicted response amplitudes originating in each layer were then compared to pharmacologically “dissected” mfERGs obtained from other studies in monkey eyes.
Results
Across the duration of the response, the amplitude of the modeled contribution from (1) the inner retina was small-to-modest, (2) the postreceptor retina was larger and contained two prominent peaks, and (3) the photoreceptor response was the largest and most closely paralleled the overall (i.e., intact) response, including late-appearing oscillations. The significance of each layer’s contribution was greatest when the absolute amplitude of that layer’s response was largest. The contribution of the inner retina was maximally significant in the interval between the prominent troughs and peaks of the intact response. The contributions of the postreceptor and photoreceptor responses were maximally significant at the prominent troughs and peaks of the intact response.
Conclusions
The results of the model were in good overall agreement with previous interpretations of the cellular contributions to the mfERG. There was also fair agreement with pharmacologically dissected monkey mfERG responses. Thus, the estimations of the contributions of the retinal layers to the mfERG so produced appeared plausible.





Similar content being viewed by others
References
Sutter EE, Tran D (1992) The field topography of ERG components in man–I. The photopic luminance response. Vision Res 32(3):433–446
Hood DC et al (2003) The multifocal electroretinogram. J Neuroophthalmol 23(3):225–235
Hood DC et al (1999) Evidence for a ganglion cell contribution to the primate electroretinogram (ERG): effects of TTX on the multifocal ERG in macaque. Vis Neurosci 16(3):411–416
Hood DC et al (1999) Identifying inner retinal contributions to the human multifocal ERG. Vision Res 39(13):2285–2291
Hood DC et al (2002) Retinal origins of the primate multifocal ERG: implications for the human response. Invest Ophthalmol Vis Sci 43(5):1673–1685
Rangaswamy NV, Hood DC, Frishman LJ (2003) Regional variations in local contributions to the primate photopic flash ERG: revealed using the slow-sequence mfERG. Invest Ophthalmol Vis Sci 44(7):3233–3247
Hare WA, Ton H (2002) Effects of APB, PDA, and TTX on ERG responses recorded using both multifocal and conventional methods in monkey. Effects of APB, PDA, and TTX on monkey ERG responses. Doc Ophthalmol 105(2):189–222
Narahashi T (1974) Chemicals as tools in the study of excitable membranes. Physiol Rev 54(4):813–889
Massey SC (1990) Cell types using glutamate as a neurotransmitter in the vertebrate retina. Prog Retin Res 9:399–425
Slaughter MM, Miller RF (1981) 2-amino-4-phosphonobutyric acid: a new pharmacological tool for retina research. Science 211(4478):182–185
Slaughter MM, Miller RF (1983) An excitatory amino acid antagonist blocks cone input to sign-conserving second-order retinal neurons. Science 219(4589):1230–1232
Wässle H et al (1990) Retinal ganglion cell density and cortical magnification factor in the primate. Vision Res 30(11):1897–1911
Curcio CA, Allen KA (1990) Topography of ganglion cells in human retina. J Comp Neurol 300(1):5–25
Huang D et al (1991) Optical coherence tomography. Science 254(5035):1178–1181
Altschwager P et al (2017) Multifocal ERG responses in subjects with a history of preterm birth. Invest Ophthalmol Vis Sci 58(5):2603–2608
Akula JD et al (2020) The Fovea in retinopathy of prematurity. Invest Ophthalmol Vis Sci 61(11):28
Hoffmann MB et al (2021) ISCEV standard for clinical multifocal electroretinography (mfERG) (2021 update). Doc Ophthalmol 142(1):5–16
Poloschek CM, Bach M (2009) The mfERG response topography with scaled stimuli: effect of the stretch factor. Doc Ophthalmol 119(1):51–58
Ramamirtham R et al (2016) Extrafoveal cone packing in eyes with a history of retinopathy of prematurity. Invest Ophthalmol Vis Sci 57(2):467–475
Li KY, Tiruveedhula P, Roorda A (2010) Intersubject variability of foveal cone photoreceptor density in relation to eye length. Invest Ophthalmol Vis Sci 51(12):6858–6867
Bennett AG, Rabbetts RB, Bennett AG (1998) Bennett and Rabbetts’ clinical visual optics, 3rd edn. Butterworth-Heinemann, Oxford, Boston, p 451
Rohatgi A (2020) WebPlotDigitizer 4.4. [cited 2021 May]; Available from: https://automeris.io/WebPlotDigitizer
Sieving PA, Murayama K, Naarendorp F (1994) Push-pull model of the primate photopic electroretinogram: a role for hyperpolarizing neurons in shaping the b-wave. Vis Neurosci 11(3):519–532
Nelson R, Kolb H (1983) Synaptic patterns and response properties of bipolar and ganglion cells in the cat retina. Vision Res 23(10):1183–1195
Raviola E, Gilula NB (1975) Intramembrane organization of specialized contacts in the outer plexiform layer of the retina. A freeze-fracture study in monkeys and rabbits. J Cell Biol 65(1):192–222
Akula JD et al (2019) Extracting the ON and OFF contributions to the full-field photopic flash electroretinogram using summed growth curves. Exp Eye Res 189:107827
Sutter EE, Bearse MA Jr (1999) The optic nerve head component of the human ERG. Vis Res 39(3):419–436
Hood DC et al (2001) The optic nerve head component of the monkey’s (Macaca mulatta) multifocal electroretinogram (mERG). Vis Res 41(16):2029–2041
Wen Y et al (2012) Relationships among multifocal electroretinogram amplitude, visual field sensitivity, and SD-OCT receptor layer thicknesses in patients with retinitis pigmentosa. Invest Ophthalmol Vis Sci 53(2):833–840
Funding
This study was funded by the Boston Children’s Hospital Ophthalmology Foundation (JDA) and The National Institutes of Health EY010597 (ABF) and EY028953 (JDA).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors delcare that they have no conflicts of interest.
Ethical approval
This study involved human participants and was approved by the ethical standards committee of Boston Children's Hospital.
Statement of human rights
This study was conducted in accordance with the 1964 Helsinki Declaration and its later ammendments.
Informed concent
Informed consent was obtained from all participants in the study.
Statement on the welfare of animals
This article does not contain any studies with animal participants.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fonseca, M.I., Nouck-a-Nwal, A., Ambrosio, L. et al. The relation of the multifocal electroretinographic response to macular layer volume. Doc Ophthalmol 145, 1–10 (2022). https://doi.org/10.1007/s10633-022-09873-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10633-022-09873-z