Abstract
Purpose
To report the 30-months’ course of macular dystrophy in a patient with genetically confirmed spinocerebellar ataxia type1 (SCA1).
Methods
Detailed ophthalmological examinations including best-corrected visual acuity (BCVA), perimetry, multimodal fundus imaging, and electrophysiological recordings were performed on a 52-year-old woman with SCA1. The number of CAG sequence repeats of the candidate gene was verified.
Results
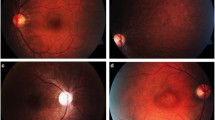
The baseline decimal BCVA was 0.2 OD and 0.3 OS. Goldman perimetry showed relative central scotomas and slight enlargements of Mariotte blind spot bilaterally. Ophthalmoscopy revealed no abnormalities in the macula and optic disk. Fundus autofluorescence (FAF) showed a circular hyperautofluorescence and round-shaped hypoautofluorescence in the macula. Optical coherence tomography (OCT) showed a loss of the interdigitation zone and ellipsoid zone (EZ) in the macula. Full-field scotopic and photopic Full-field electroretinograms (ERGs) were normal, and multifocal ERGs were decreased in the central area. After 30 months, the BCVA had not changed, but the FAF showed a spark-like hypoautofluorescence in the macula. The abnormal area of the EZ had expanded toward the periphery, and the rate of EZ loss was 199.7%/year OD and 206.8%/year OS. Genetic examinations revealed an increase in the number of heterozygous CAG repeats in the ATXN1 gene, and the CAG repeat number of the mutant allele ranged from 43 to 48.
Conclusions
The full-field scotopic and photopic ERGs were normal. The mfERGs were significantly smaller in the central region. OCT demonstrated bilateral photoreceptor atrophy in the macula, and the rate of EZ loss was more rapid than in other macular dystrophies. Spark-like hypoautofluorescence appeared during the course of the disease process which might be a specific feature of SCA1-related retinopathy.






Similar content being viewed by others
References
Schöls L, Bauer P, Schmidt T, Schulte T, Riess O (2004) Autosomal dominant cerebellar ataxias: clinical features, genetics, and pathogenesis. Lancet Neurol 3(5):291–304. https://doi.org/10.1016/s1474-4422(04)00737-9
Abe T, Tsuda T, Yoshida M, Wada Y, Kano T, Itoyama Y, Tamai M (2000) Macular degeneration associated with aberrant expansion of trinucleotide repeat of the SCA7 gene in 2 Japanese families. Arch Ophthalmol 118(10):1415–1421. https://doi.org/10.1001/archopht.118.10.1415
Katagiri S, Hayashi T, Takeuchi T, Yamada H, Gekka T, Kawabe K, Kurita A, Tsuneoka H (2015) Somatic instability of expanded CAG repeats of ATXN7 in Japanese patients with spinocerebellar ataxia type 7. Doc Ophthalmol 130(3):189–195. https://doi.org/10.1007/s10633-015-9488-8
Hugosson T, Gränse L, Ponjavic V, Andréasson S (2009) Macular dysfunction and morphology in spinocerebellar ataxia type 7 (SCA 7). Ophthalmic Genet 30(1):1–6. https://doi.org/10.1080/13816810802454081
Levinson JD, Yan J, Lambert SR, Shankar SP (2016) Multimodal imaging of a family with spinocerebellar ataxia type 7 demonstrating phenotypic variation and progression of retinal degeneration. Retinal Cases Brief Rep 10(3):267–272. https://doi.org/10.1097/icb.0000000000000248
Park JY, Wy SY, Joo K, Woo SJ (2019) Spinocerebellar ataxia type 7 with RP1L1-negative occult macular dystrophy as retinal manifestation. Ophthalmic Genet 40(3):282–285. https://doi.org/10.1080/13816810.2019.1633548
Orr HT, Chung MY, Banfi S, Kwiatkowski TJ Jr, Servadio A, Beaudet AL, McCall AE, Duvick LA, Ranum LP, Zoghbi HY (1993) Expansion of an unstable trinucleotide CAG repeat in spinocerebellar ataxia type 1. Nat Genet 4(3):221–226. https://doi.org/10.1038/ng0793-221
Whaley NR, Fujioka S, Wszolek ZK (2011) Autosomal dominant cerebellar ataxia type I: a review of the phenotypic and genotypic characteristics. Orphanet J Rare Dis 6:33. https://doi.org/10.1186/1750-1172-6-33
Abe T, Abe K, Aoki M, Itoyama Y, Tamai M (1997) Ocular changes in patients with spinocerebellar degeneration and repeated trinucleotide expansion of spinocerebellar ataxia type 1 gene. Arch Ophthalmol 115(2):231–236. https://doi.org/10.1001/archopht.1997.01100150233013
Perretti A, Santoro L, Lanzillo B, Filla A, De Michele G, Barbieri F, Martino G, Ragno M, Cocozza S, Caruso G (1996) Autosomal dominant cerebellar ataxia type I: multimodal electrophysiological study and comparison between SCA1 and SCA2 patients. J Neurol Sci 142(1–2):45–53. https://doi.org/10.1016/0022-510x(96)00140-2
Abele M, Bürk K, Andres F, Topka H, Laccone F, Bösch S, Brice A, Cancel G, Dichgans J, Klockgether T (1997) Autosomal dominant cerebellar ataxia type I. Nerve conduction and evoked potential studies in families with SCA1, SCA2 and SCA3. Brain: J Neurol 120(12):2141–2148. https://doi.org/10.1093/brain/120.12.2141
Saito Y, Matsumura K, Shimizu S, Ichikawa Y, Ochiai K, Goto J, Tsuji S, Shimizu T (2006) Pigmentary macular dystrophy in spinocerebellar ataxia type 1. J Neurol Neurosurg Psychiatry 77(11):1293. https://doi.org/10.1136/jnnp.2006.092676
Pula JH, Towle VL, Staszak VM, Cao D, Bernard JT, Gomez CM (2011) Retinal nerve fibre layer and macular thinning in spinocerebellar ataxia and cerebellar multisystem atrophy. Neuro-ophthalmology 35(3):108–114. https://doi.org/10.3109/01658107.2011.580898
Stricker S, Oberwahrenbrock T, Zimmermann H, Schroeter J, Endres M, Brandt AU, Paul F (2011) Temporal retinal nerve fiber loss in patients with spinocerebellar ataxia type 1. PLoS ONE 6(7):e23024. https://doi.org/10.1371/journal.pone.0023024
Thurtell MJ, Biousse V, Newman NJ (2011) Rod-cone dystrophy in spinocerebellar ataxia type 1. Arch Ophthalmol 129(7):956–958. https://doi.org/10.1001/archophthalmol.2011.172
Lebranchu P, Le Meur G, Magot A, David A, Verny C, Weber M, Milea D (2013) Maculopathy and spinocerebellar ataxia type 1: a new association? J Neuro-ophthalmol 33(3):225–231. https://doi.org/10.1097/WNO.0b013e31828d4add
Vaclavik V, Borruat FX, Ambresin A, Munier FL (2013) Novel maculopathy in patients with spinocerebellar ataxia type 1 autofluorescence findings and functional characteristics. JAMA Ophthalmol 131(4):536–538. https://doi.org/10.1001/jamaophthalmol.2013.1127
Nishiguchi KM, Aoki M, Nakazawa T, Abe T (2019) Macular degeneration as a common cause of visual loss in spinocerebellar ataxia type 1 (SCA1) patients. Ophthalmic Genet 40(1):49–53. https://doi.org/10.1080/13816810.2019.1571614
Schöls L, Linnemann C, Globas C (2008) Electrophysiology in spinocerebellar ataxias: spread of disease and characteristic findings. Cerebellum 7(2):198–203. https://doi.org/10.1007/s12311-008-0024-1
Cai CX, Light JG, Handa JT (2018) Quantifying the rate of ellipsoid zone loss in stargardt disease. Am J Ophthalmol 186:1–9. https://doi.org/10.1016/j.ajo.2017.10.032
Tanna P, Georgiou M, Strauss RW, Ali N, Kumaran N, Kalitzeos A, Fujinami K, Michaelides M (2019) Cross-sectional and longitudinal assessment of the ellipsoid zone in childhood-onset stargardt disease. Transl Vis Sci Technol 8(2):1. https://doi.org/10.1167/tvst.8.2.1
McCulloch DL, Marmor MF, Brigell MG, Hamilton R, Holder GE, Tzekov R, Bach M (2015) ISCEV Standard for full-field clinical electroretinography (2015 update). Doc Ophthalmol 130(1):1–12. https://doi.org/10.1007/s10633-014-9473-7
Hood DC, Bach M, Brigell M, Keating D, Kondo M, Lyons JS, Marmor MF, McCulloch DL, Palmowski-Wolfe AM (2012) ISCEV standard for clinical multifocal electroretinography (mfERG) (2011 edition). Doc Ophthalmol 124(1):1–13. https://doi.org/10.1007/s10633-011-9296-8
Nakamura N, Tsunoda K, Mizuno Y, Usui T, Hatase T, Ueno S, Kuniyoshi K, Hayashi T, Katagiri S, Kondo M, Kameya S, Yoshitake K, Fujinami K, Iwata T, Miyake Y (2019) Clinical stages of occult macular dystrophy based on optical coherence tomographic findings. Invest Ophthalmol Vis Sci 60(14):4691–4700. https://doi.org/10.1167/iovs.19-27486
Miyake Y, Ichikawa K, Shiose Y, Kawase Y (1989) Hereditary macular dystrophy without visible fundus abnormality. Am J Ophthalmol 108(3):292–299
Miyake Y, Tsunoda K (2015) Occult macular dystrophy. Jpn J Ophthalmol 59(2):71–80. https://doi.org/10.1007/s10384-015-0371-7
Fujinami K, Kameya S, Kikuchi S, Ueno S, Kondo M, Hayashi T, Shinoda K, Machida S, Kuniyoshi K, Kawamura Y, Akahori M, Yoshitake K, Katagiri S, Nakanishi A, Sakuramoto H, Ozawa Y, Tsubota K, Yamaki K, Mizota A, Terasaki H, Miyake Y, Iwata T, Tsunoda K (2016) Novel RP1L1 variants and genotype-photoreceptor microstructural phenotype associations in Cohort of Japanese patients with occult macular dystrophy. Invest Ophthalmol Vis Sci 57(11):4837–4846. https://doi.org/10.1167/iovs.16-19670
Akahori M, Tsunoda K, Miyake Y, Fukuda Y, Ishiura H, Tsuji S, Usui T, Hatase T, Nakamura M, Ohde H, Itabashi T, Okamoto H, Takada Y, Iwata T (2010) Dominant mutations in RP1L1 are responsible for occult macular dystrophy. Am J Hum Genet 87(3):424–429
Tsunoda K, Usui T, Hatase T, Yamai S, Fujinami K, Hanazono G, Shinoda K, Ohde H, Akahori M, Iwata T, Miyake Y (2012) Clinical characteristics of occult macular dystrophy in family with mutation of Rp1l1 GENE. Retina J Retinal Vitreous Dis 32(6):1135–1147. https://doi.org/10.1097/Iae.0b013e318232c32e
Fujinami K, Tsunoda K, Hanazono G, Shinoda K, Ohde H, Miyake Y (2011) Fundus autofluorescence in autosomal dominant occult macular dystrophy. Arch Ophthalmol 129(5):597–602
Birch DG, Locke KG, Wen Y, Locke KI, Hoffman DR, Hood DC (2013) Spectral-domain optical coherence tomography measures of outer segment layer progression in patients with X-linked retinitis pigmentosa. JAMA Ophthalmol 131(9):1143–1150. https://doi.org/10.1001/jamaophthalmol.2013.4160
Sujirakul T, Lin MK, Duong J, Wei Y, Lopez-Pintado S, Tsang SH (2015) Multimodal imaging of central retinal disease progression in a 2-year mean follow-up of retinitis pigmentosa. Am J Ophthalmol 160(4):786–798. https://doi.org/10.1016/j.ajo.2015.06.032
Tee JJL, Yang Y, Kalitzeos A, Webster A, Bainbridge J, Michaelides M (2019) Natural history study of retinal structure, progression, and symmetry using ellipzoid zone metrics in RPGR-associated retinopathy. Am J Ophthalmol 198:111–123. https://doi.org/10.1016/j.ajo.2018.10.003
Servadio A, Koshy B, Armstrong D, Antalffy B, Orr HT, Zoghbi HY (1995) Expression analysis of the ataxin-1 protein in tissues from normal and spinocerebellar ataxia type 1 individuals. Nat Genet 10(1):94–98. https://doi.org/10.1038/ng0595-94
Duyao MP, Auerbach AB, Ryan A, Persichetti F, Barnes GT, McNeil SM, Ge P, Vonsattel JP, Gusella JF, Joyner AL et al (1995) Inactivation of the mouse Huntington’s disease gene homolog Hdh. Science 269(5222):407–410. https://doi.org/10.1126/science.7618107
Lam YC, Bowman AB, Jafar-Nejad P, Lim J, Richman R, Fryer JD, Hyun ED, Duvick LA, Orr HT, Botas J, Zoghbi HY (2006) ATAXIN-1 interacts with the repressor Capicua in its native complex to cause SCA1 neuropathology. Cell 127(7):1335–1347. https://doi.org/10.1016/j.cell.2006.11.038
Buijsen RAM, Toonen LJA, Gardiner SL, van Roon-Mom WMC (2019) Genetics, mechanisms, and therapeutic progress in polyglutamine spinocerebellar ataxias. Neurotherapeutics 16(2):263–286. https://doi.org/10.1007/s13311-018-00696-y
Kourkouta E, Weij R, González-Barriga A, Mulder M, Verheul R, Bosgra S, Groenendaal B, Puoliväli J, Toivanen J, van Deutekom JCT, Datson NA (2019) Suppression of mutant protein expression in SCA3 and SCA1 mice using a CAG repeat-targeting antisense oligonucleotide. Mol Ther Nucleic Acids 17:601–614. https://doi.org/10.1016/j.omtn.2019.07.004
Friedrich J, Kordasiewicz HB, O’Callaghan B, Handler HP, Wagener C, Duvick L, Swayze EE, Rainwater O, Hofstra B, Benneyworth M, Nichols-Meade T, Yang P, Chen Z, Ortiz JP, Clark HB, Öz G, Larson S, Zoghbi HY, Henzler C, Orr HT (2018) Antisense oligonucleotide-mediated ataxin-1 reduction prolongs survival in SCA1 mice and reveals disease-associated transcriptome profiles. JCI Insight. https://doi.org/10.1172/jci.insight.123193
Cideciyan AV, Jacobson SG, Drack AV, Ho AC, Charng J, Garafalo AV, Roman AJ, Sumaroka A, Han IC, Hochstedler MD, Pfeifer WL, Sohn EH, Taiel M, Schwartz MR, Biasutto P, Wit W, Cheetham ME, Adamson P, Rodman DM, Platenburg G, Tome MD, Balikova I, Nerinckx F, Zaeytijd J, Van Cauwenbergh C, Leroy BP, Russell SR (2019) Effect of an intravitreal antisense oligonucleotide on vision in Leber congenital amaurosis due to a photoreceptor cilium defect. Nat Med 25(2):225–228. https://doi.org/10.1038/s41591-018-0295-0
Acknowledgements
We thank the patient for participation in this study. We thank Professor Emeritus Duco Hamasaki of the Bascom Palmer Eye Institute, University of Miami School of Medicine, Mami, FL, for discussions and editing our manuscript.
Funding
This study is supported by research grants from the Japan Agency for Medical Research and Development (AMED), the Ministry of Health, Labor and Welfare, and Japan (18ek0109282h0002 to TI) and National Hospital Organization Network Research Fund (H30-NHO-Sensory Organs-03 to KF, KT). The authors have no proprietary or commercial interest in any materials discussed in this article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Author KT has received financial research support from Astellas and speaker honorarium from Novartis, Santen, and Senju. Other authors have no financial disclosure.
Informed consent
Informed consent was obtained from the patient.
Statement of human rights
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee (Review Board/Ethics Committee of the National Institute of Sensory Organs, National Hospital Organization, Tokyo Medical Center, Reference: R18-029) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Statement on the welfare of animals
This article does not contain any studies with animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hirose, A., Katagiri, S., Hayashi, T. et al. Progress of macular atrophy during 30 months’ follow-up in a patient with spinocerebellar ataxia type1 (SCA1). Doc Ophthalmol 142, 87–98 (2021). https://doi.org/10.1007/s10633-020-09782-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10633-020-09782-z