Abstract
Purpose
To study how rod- and cone-driven responses depend on stimulus size in normal subjects and patients with retinitis pigmentosa (RP), and to show that comparisons between responses to full-field (FF) and smaller stimuli can be useful in diagnosing and monitoring disorders of the peripheral retina without the need for lengthy dark adaptation periods.
Method
The triple silent substitution technique was used to isolate L-cone-, M-cone- and rod-driven ERGs with 19, 18 and 33% photoreceptor contrasts, respectively, under identical mean luminance conditions. Experiments were conducted on five normal subjects and three RP patients. ERGs on control subjects were recorded at nine different temporal frequencies (between 2 and 60 Hz) for five different stimulus sizes: FF, 70°, 60°, 50° and 40° diameter circular stimuli. Experiments on RP patients involved rod- and L-cone-driven ERG measurements with FF and 40° stimuli at 8 and 48 Hz. Response amplitudes were defined as those of the first harmonic component after Fourier analysis.
Results
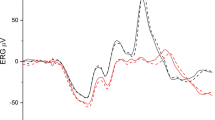
In normal subjects, rod-driven responses displayed a fundamentally different behavior than cone-driven responses, particularly at low temporal frequencies. At low and intermediate temporal frequencies (≤ 12 Hz), rod-driven signals increased by a factor of about four when measured with smaller stimuli. In contrast, L- and M-cone-driven responses in this frequency region did not change substantially with stimulus size. At high temporal frequencies (≥ 24 Hz), both rod- and cone-driven response amplitudes decreased with decreasing stimulus size. Signals obtained from rod-isolating stimuli under these conditions are likely artefactual. Interestingly, in RP patients, both rod-driven and L-cone-driven ERGs were similar using 40° and FF stimuli.
Conclusion
The increased responses with smaller stimuli in normal subjects to rod-isolating stimuli indicate that a fundamentally different mechanism drives the ERGs in comparison with the cone-driven responses. We propose that the increased responses are caused by stray light stimulating the peripheral retina, thereby allowing peripheral rod-driven function to be studied using the triple silent substitution technique at photopic luminances. The method is effective in studying impaired peripheral rod- and cone- function in RP patients.











Similar content being viewed by others
References
Stockman A, MacLeod DIA, Johnson NE (1993) Spectral sensitivities of the human cones. J Opt Soc Am A 10:2491–2521
Estevez O, Spekreijse H (1982) The “silent substitution” method in visual research. Vis Res 22(6):681–691
Estevez O, Spekreijse H (1974) A spectral compensation method for determining the flicker characteristics of the human colour mechanisms. Vis Res 14(9):823–830
Donner KO, Rushton WAH (1959) Retinal stimulation by light substitution. J Physiol 149:288–302
Shapiro AG, Pokorny J, Smith VC (1996) Cone-rod receptor spaces with illustrations that use CRT phosphor and light-emitting-diode spectra. J Opt Soc Am A 13(12):2319–2328
Kremers J (2003) The assessment of L- and M-cone specific electroretinographical signals in the normal and abnormal human retina. Prog Retin Eye Res 22(5):579–605
Kremers J, Scholl HPN (2001) Rod-/L-cone and rod-/M-cone interactions in electroretinograms at different temporal frequencies. Vis Neurosci 18:339–351
Ripamonti C, Woo WL, Crowther E, Stockman A (2009) The S-cone contribution to luminance depends on the M- and L-cone adaptation levels: silent surrounds? J Vis 9(3):1–16
Challa NK, McKeefry D, Parry NR, Kremers J, Murray IJ, Panorgias A (2010) L- and M-cone input to 12 and 30 Hz flicker ERGs across the human retina. Ophthalmic Physiol Opt 30(5):503–510
Jacob MM, Pangeni G, Gomes BD, Souza GS, Filho MDS, Silveira LC, Maguire J, Parry NR, McKeefry DJ, Kremers J (2015) The spatial properties of L- and m-cone inputs to electroretinograms that reflect different types of post-receptoral processing. PLoS ONE 10(3):e0121218
Hagstrom SA, Neitz J, Meitz M (1997) Ratio of M/L pigment gene expression decreases with retinal eccentricity. In: Cavonius CR (ed) Colour vision deficiencies XIII. Documenta Ophthalmologica Proceedings Series vol 59. Springer, Netherlands, pp 59–65
Martins CM, Tsai T, Barboni MT, da Costa MF, Nagy B, Ventura DF, Kremers J (2016) The influence of stimulus size on heterochromatic modulation electroretinograms. J Vis 16(8):1–11
Maguire J, Parry NRA, Kremers J, Kommanapalli D, Murray IJ, Mckeefry D (2016) Rod electroretinograms elicited by silent substitution stimuli for the light-adapted human eye. Trans Vis Sci Tech 5(4):13. https://doi.org/10.1167/tvst.5.4.13
Maguire J, Parry NR, Kremers J, Murray IJ, McKeefry D (2017) The morphology of human rod ERGs obtained by silent substitution stimulation. Doc Ophthalmol 134:11–24
Berson EL, Gouras P, Gunkel RD (1968) Rod responses in retinitis pigmentosa, dominantly inherited. Arch Ophthalmol 80(1):58–67
Berson EL, Gouras P, Gunkel RD, Marianthopoulos NC (1969) Rod and cone responses in sex-linked retinitis pigmentosa. Arch Ophthalmol 81(2):215–225
Scholl HPN, Langrová H, Weber BH, Zrenner E, Apfelstedt-Sylla E (2001) Clinical electrophysiology of two rod pathways: normative values and clinical application. Graefes Arch Clin Exp Ophthalmol 239(2):71–80
Marmor MF, Zrenner E (1995) Standard for clinical electrophysiology (1994 update). Doc Ophthalmol 89:199–210
Meigen T, Bach M (1999) On the statistical significance of electrophysiological steady-state responses. Doc Ophthalmol 98(3):207–232
Kremers J, Link B (2008) Electroretinographic responses that may reflect activity of parvo- and magnocellular post-receptoral visual pathways. J Vis 8(15):11–14
Kommanapalli D, Murray IJ, Kremers J, Parry NR, McKeefry DJ (2014) Temporal characteristics of L- and M-cone isolating steady-state electroretinograms. J Opt Soc Am A 31(4):A113–A120
Kremers J, Pangeni G (2012) Electroretinographic responses to photoreceptor specific sin wave modulation. J Opt Soc Am A 29:306–313
Tsai TI, Jacob MM, McKeefry D, Murray IJ, Parry NR, Kremers J (2016) Spatial properties of L- and M-cone driven incremental (On-) and decremental (Off-) electroretinograms: evidence for the involvement of multiple post-receptoral mechanisms. J Opt Soc Am A 33(3):A1–11
Huchzermeyer C, Kremers J (2017) Perifoveal S-cone and rod-driven temporal contrast sensitivities at different retinal illuminances. J Opt Soc Am A 34(2):171–183
Gouras P, Carr RE (1964) Electrophysiological studies in early retinitis pigmentosa. Arch Ophthalmol 72(1):104–110
Scholl HPN, Kremers J (2000) Large phase differences between L-cone and M-cone driven electroretinograms in retinitis pigmentosa. Invest Ophthalmol Vis Sci 41:3225–3233
McCulloch DL, Marmor MF, Brigell MG, Hamilton R, Holder GE, Tzekov R, Bach M (2015) ISCEV standard for full-field clinical electroretinography (2015 update). Doc Ophthalmol 130(1):1–12
Jacobson SG, Roman AJ, Aleman TS, Sumaroka A, Herrera W, Windsor EA, Atkinson LA, Schwartz SB, Steinberg JD, Cideciyan AV (2010) Normal central retinal function and structure preserved in retinitis pigmentosa. Invest Ophthalmol Vis Sci 51(2):1079–1085
Holopigian K, Seiple W, Greenstein VC, Hood DC, Carr RE (2001) Local cone and rod system function in patients with retinitis pigmentosa. Inves Ophthalmol Vis Sci 42(3):779–788
Acknowledgements
The present work was performed in (partial) fulfillment of the requirements for obtaining the degree “Dr. rer. biol. hum” for AJA. NRAP’s participation was facilitated by the Manchester Biomedical Research Center and the Greater Manchester Comprehensive Local Research Network.
Funding
Bundesministerium fur Bildung und Forschung (BMBF) provided financial support in the form of project funding to JK (Grant#: 01DN14009). Deutsche Forschungsgemeinschaft (DFG) provided financial support in the form of project funding to JK (Grant#: KR1317/13-1). The sponsor had no role in the design or conduct of this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership or other equity interest; and expert testimony or patent-licensing arrangements) or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study. Additional informed consent was obtained from all individual participants for whom identifying information is included in this article.
Statement of human rights
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Statement on the welfare of animals
This article does not contain any studies with animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Aher, A.J., McKeefry, D.J., Parry, N.R.A. et al. Rod- versus cone-driven ERGs at different stimulus sizes in normal subjects and retinitis pigmentosa patients. Doc Ophthalmol 136, 27–43 (2018). https://doi.org/10.1007/s10633-017-9619-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10633-017-9619-5