Abstract
Purpose
To establish reproducibility of multifocal visual evoked potential (mfVEP) and traditional pattern-reversal VEP (tVEP) in normals and relapsing–remitting multiple sclerosis (RRMS).
Methods
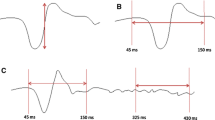
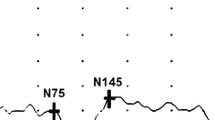
mfVEP (60-sector dartboard) was recorded twice within a month in 40 normals and 40 RRMS patients [25 eyes with last optic neuritis (ON) ≥6 months, 34 non-ON]. mfVEP amplitude and latency (ms) were calculated as mean logSNR and median relative latency, respectively, for all 60 sectors (global) and 9 regions. tVEP was recorded (15′, 60′ and 120′ checks) in subsets of 34 normals and 30 RRMS patients. tVEP N75–P100 amplitude (µV) and P100 latency (ms) were obtained. Reproducibility was evaluated using intraclass correlation coefficient (ICC) and test–retest variability (TRV). ICC ≥ 0.75 was considered good.
Results
ICCs for global and regional mfVEP were >0.80 in all groups. ICCs for tVEP were >0.75 for all except latency in ON (0.52–0.68). For mfVEP or tVEP, TRV for amplitude was similar among all groups; TRV for latency (ms) was larger in ON (5.3 for mfVEP global, 10.3 for 60′ tVEP) compared with non-ON (3.1, 8.3) and normal (2.3, 5.7) (p < 0.05 for all). When tVEP was analyzed using similar methods as mfVEP (logSNR and relative latency), mfVEP global measures showed better ICC and TRV than tVEP in all groups.
Conclusions
mfVEP and tVEP showed good reproducibility in normals and RRMS. TRV for mfVEP latency was larger in ON than normal or non-ON. mfVEP global latency’s TRV was about half the respective values for tVEP in all groups, due to averaging of multiple responses.




Similar content being viewed by others
References
Dutta R, Trapp BD (2011) Mechanisms of neuronal dysfunction and degeneration in multiple sclerosis. Prog Neurobiol 93(1):1–12
Irvine KA, Blakemore WF (2008) Remyelination protects axons from demyelination-associated axon degeneration. Brain 131(Pt 6):1464–1477
Syc SB, Saidha S, Newsome SD, Ratchford JN, Levy M, Ford E, Crainiceanu CM, Durbin MK, Oakley JD, Meyer SA, Frohman EM, Calabresi PA (2012) Optical coherence tomography segmentation reveals ganglion cell layer pathology after optic neuritis. Brain 135(Pt 2):521–533
Narayanan D, Cheng H, Bonem KN, Saenz R, Tang RA, Frishman LJ (2014) Tracking changes over time in retinal nerve fiber layer and ganglion cell-inner plexiform layer thickness in multiple sclerosis. Mult Scler 20(10):1331–1341
Jones SJ, Brusa A (2003) Neurophysiological evidence for long-term repair of MS lesions: implications for axon protection. J Neurol Sci 206(2):193–198
Klistorner A, Arvind H, Garrick R, Graham SL, Paine M, Yiannikas C (2010) Interrelationship of optical coherence tomography and multifocal visual-evoked potentials after optic neuritis. Investig Ophthalmol Vis Sci 51(5):2770–2777
Klistorner A, Arvind H, Garrick R, Yiannikas C, Paine M, Graham SL (2010) Remyelination of optic nerve lesions: spatial and temporal factors. Mult Scler 16(7):786–795
Hood DC, Greenstein VC (2003) Multifocal VEP and ganglion cell damage: applications and limitations for the study of glaucoma. Prog Retin Eye Res 22(2):201–251
Klistorner A, Fraser C, Garrick R, Graham S, Arvind H (2008) Correlation between full-field and multifocal VEPs in optic neuritis. Doc Ophthalmol 116(1):19–27
Fraser CL, Klistorner A, Graham SL, Garrick R, Billson FA, Grigg JR (2006) Multifocal visual evoked potential analysis of inflammatory or demyelinating optic neuritis. Ophthalmology 113(2):323.e1–323.e2
Laron M, Cheng H, Zhang B, Schiffman JS, Tang RA, Frishman LJ (2009) Assessing visual pathway function in multiple sclerosis patients with multifocal visual evoked potentials. Mult Scler 15(12):1431–1441
Grover LK, Hood DC, Ghadiali Q, Grippo TM, Wenick AS, Greenstein VC, Behrens MM, Odel JG (2008) A comparison of multifocal and conventional visual evoked potential techniques in patients with optic neuritis/multiple sclerosis. Doc Ophthalmol 117(2):121–128
Laron M, Cheng H, Zhang B, Schiffman JS, Tang RA, Frishman LJ (2010) Comparison of multifocal visual evoked potential, standard automated perimetry and optical coherence tomography in assessing visual pathway in multiple sclerosis patients. Mult Scler 16(4):412–426
Hood DC, Odel JG, Zhang X (2000) Tracking the recovery of local optic nerve function after optic neuritis: a multifocal VEP study. Investig Ophthalmol Vis Sci 41(12):4032–4038
Yang EB, Hood DC, Rodarte C, Zhang X, Odel JG, Behrens MM (2007) Improvement in conduction velocity after optic neuritis measured with the multifocal VEP. Investig Ophthalmol Vis Sci 48(2):692–698
Fortune B, Demirel S, Zhang X, Hood DC, Johnson CA (2006) Repeatability of normal multifocal VEP: implications for detecting progression. J Glaucoma 15(2):131–141
Chen CS, Hood DC, Zhang X, Karam EZ, Liebmann JM, Ritch R, Thienprasiddhi P, Greenstein VC (2003) Repeat reliability of the multifocal visual evoked potential in normal and glaucomatous eyes. J Glaucoma 12(5):399–408
Sriram P, Klistorner A, Arvind H, Graham SL (2012) Reproducibility of multifocal VEP latency using different stimulus presentations. Doc Ophthalmol 125(1):43–49
Compston A, Coles A (2008) Multiple sclerosis. Lancet 372(9648):1502–1517
Wall M, Johnson CA, Kutzko KE, Nguyen R, Brito C, Keltner JL (1998) Long- and short-term variability of automated perimetry results in patients with optic neuritis and healthy subjects. Arch Ophthalmol 116(1):53–61
Chauhan BC, Johnson CA (1999) Test–retest variability of frequency-doubling perimetry and conventional perimetry in glaucoma patients and normal subjects. Investig Ophthalmol Vis Sci 40(3):648–656
Polman CH, Wolinsky JS, Reingold SC (2005) Multiple sclerosis diagnostic criteria: three years later. Mult Scler 11(1):5–12
Fortune B, Zhang X, Hood DC, Demirel S, Johnson CA (2004) Normative ranges and specificity of the multifocal VEP. Doc Ophthalmol 109(1):87–100
Hood DC, Ohri N, Yang EB, Rodarte C, Zhang X, Fortune B, Johnson CA (2004) Determining abnormal latencies of multifocal visual evoked potentials: a monocular analysis. Doc Ophthalmol 109(2):189–199
Odom JV, Bach M, Brigell M, Holder GE, McCulloch DL, Tormene AP, Vaegan (2009) ISCEV standard for clinical visual evoked potentials (2009 update). Doc Ophthalmol 120(1):111–119
Grippo TM, Hood DC, Kanadani FN, Ezon I, Greenstein VC, Liebmann JM, Ritch R (2006) A comparison between multifocal and conventional VEP latency changes secondary to glaucomatous damage. Investig Ophthalmol Vis Sci 47(12):5331–5336
Lee J, Koh D, Ong CN (1989) Statistical evaluation of agreement between two methods for measuring a quantitative variable. Comput Biol Med 19(1):61–70
Bland JM, Altman DG (1996) Measurement error. BMJ 313(7059):744
Budenz DL, Fredette MJ, Feuer WJ, Anderson DR (2008) Reproducibility of peripapillary retinal nerve fiber thickness measurements with stratus OCT in glaucomatous eyes. Ophthalmology 115(4):661–666.e4
Wangsupadilok B, Greenstein VC, Kanadani FN, Grippo TM, Liebmann JM, Ritch R, Hood DC (2009) A method to detect progression of glaucoma using the multifocal visual evoked potential technique. Doc Ophthalmol 118(2):139–150
Bjerre A, Grigg JR, Parry NR, Henson DB (2004) Test–retest variability of multifocal visual evoked potential and SITA standard perimetry in glaucoma. Investig Ophthalmol Vis Sci 45(11):4035–4040
Hammond SR, MacCallum S, Yiannikas C, Walsh JC, McLeod JG (1987) Variability on serial testing of pattern reversal visual evoked potential latencies from full- field, half-field and foveal stimulation in control subjects. Electroencephalogr Clin Neurophysiol 66(4):401–408
Thomae E, Niklas A, Sebraoui H, Baum P, Wagner A, Then Bergh F (2010) Improving test–retest variability of visual-evoked responses in multiple sclerosis: implications for trial design. J Clin Neurophysiol 27(4):270–273
McAlinden C, Khadka J, Pesudovs K (2011) Statistical methods for conducting agreement (comparison of clinical tests) and precision (repeatability or reproducibility) studies in optometry and ophthalmology. Ophthalmic Physiol Opt 31(4):330–338
Acknowledgments
The authors would like to thank Dr. Courtney Perry for her help in collecting some of the normal subject’s data. This study was supported by NIH P30 EY 007551, NIH T35 EY 007088, Fight for Sight summer student fellowship and the Minnie Flaura Turner memorial fund for impaired vision research.
Conflict of interest
The authors declare that they have no conflicts of interest and have full control of all primary data that could be reviewed by the journal if needed.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Narayanan, D., Cheng, H., Tang, R.A. et al. Reproducibility of multifocal visual evoked potential and traditional visual evoked potential in normal and multiple sclerosis eyes. Doc Ophthalmol 130, 31–41 (2015). https://doi.org/10.1007/s10633-014-9467-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10633-014-9467-5