Abstract
Patients with gastroparesis (Gp) often have diets deficient in calories, electrolytes, and vitamins. Vitamin D levels have been reported to be low in some patients with Gp but has not been systematically studied.
Aims
To determine vitamin D levels and relationships among symptoms, gastric emptying and gastric myoelectrical activity (GMA) in patients with symptoms of Gp.
Methods
25-hydroxy-vitamin D was measured in patients at enrollment in the Gastroparesis Clinical Consortium Registry. Gastroparesis Cardinal Symptoms Index (GCSI), gastric emptying, and GMA before and after water load satiety test (WLST) were measured. GMA, expressed as percentage distribution of activity in normal and dysrhythmic ranges, was recorded using electrogastrography.
Results
Overall, vitamin D levels were low (< 30 ng/ml) in 288 of 513 (56.1%) patients with symptoms of Gp (206 of 376 (54.8%) patients with delayed gastric emptying (Gp) and 82 of 137 (59.9%) patients with symptoms of Gp and normal gastric emptying). Low vitamin D levels were associated with increased nausea and vomiting (P < 0.0001), but not with fullness or bloating subscores. Low vitamin D levels in patients with Gp were associated with greater meal retention at four hours (36% retention) compared with Gp patients with normal vitamin D levels (31% retention; P = 0.05). Low vitamin D in patients with normal gastric emptying was associated with decreased normal 3 cpm GMA before (P = 0.001) and increased tachygastria after WLST (P = 0.01).
Conclusions
Low vitamin D levels are present in half the patients with symptoms of gastroparesis and are associated with nausea and vomiting and gastric neuromuscular dysfunction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Patients with gastroparesis (Gp) often have diets deficient in calories, electrolytes, and vitamins, including vitamin D [1, 2]. Vitamin D deficiency can occur from several causes including decreased dietary intake and/or absorption as well as decreased sun exposure. Vitamin D levels have been reported to be low in some patients with Gp. However, the relationships of vitamin D deficiency to symptoms of gastroparesis and gastric neuromuscular dysfunction in Gp has not been studied.
Extra-skeletal effects of vitamin D may have pathophysiological relevance for several aspects of neuromuscular disorders like Gp. In patients with Gp, interstitial cells of Cajal (ICCs), the pacemaker cells of the stomach, are reduced [4, 7, 10, 11]. Patients have decreased 3 cpm gastric myoelectric activity (GMA) and increased dysrhythmic gastric myoelectrical activity (GMA), e.g., tachygastria and bradygastria [7, 8]. Loss of ICCs in the corpus and antrum of patients with Gp is related, in part, to increased inflammatory M1 macrophages that replace M2 macrophages [10, 11]. Vitamin D may affect the transformation of M2 macrophages to M1 macrophages in chronic inflammatory diseases of the gastrointestinal tract [5, 6]. Low vitamin D is also associated with carotid body dysfunction and postural orthostatic tachycardia syndrome (POTS) [12]. A subset of patients with Gp with nausea and vomiting have autonomic nervous system (ANS) dysfunction, including POTS [13,14,15]. Vitamin D replenishment decreased symptoms in patients with orthostatic intolerance and nausea and vomiting during head up tilt table tests [15].
The primary aim of this study was to determine the prevalence of low vitamin D levels in patients with Gp and to explore relationships among vitamin D levels, symptoms, gastric emptying rate and GMA in response to the water load satiety test (WLST). The study was performed in patients in the NIDDK Gastroparesis Registry that enrolled patients with symptoms of Gp (both patients with delayed gastric emptying (Gp), as well as patients with normal gastric emptying). Thus, the secondary aim of this study was to explore relationships among vitamin D levels, symptoms, gastric emptying rate and GMA in response to WLST in patients with symptoms of Gp but normal gastric emptying.
Methods
Patients
The NIH Gastroparesis Clinical Research Consortium Gastroparesis Registry 2 (GpR2) (ClinicalTrials.gov Identifier: NCT01696747) was implemented as an observational study of patients with symptoms of Gp. Patients 18 years or older were enrolled at seven tertiary clinical centers in the U.S. and had symptoms of nausea, vomiting, abdominal pain, bloating, and/or early satiety for at least 12 weeks. Patients had delayed or normal gastric emptying of a solid test meal by scintigraphy [16], and normal upper endoscopy. Informed consent was obtained prior to enrollment for this study approved by the IRB of each center. Patients underwent history and physical examination, questionnaires including the Gastroparesis Cardinal Symptoms index (GCSI) [17] and the gastroduodenal disorder section of the Rome III Diagnostic Questionnaire for Adult Functional GI Disorders [18], electrogastrography with water load testing [19,20,21,22,23]. Routine laboratory tests were obtained at enrollment including serum 25-hydroxy vitamin D levels. Normal vitamin D levels were defined as ≥ 30 ng/mL. Low vitamin D levels were defined as 25-hydroxy vitamin D levels < 30 ng/mL with deficient 25-hydroxy vitamin D levels < 20 ng/mL and insufficient levels defined as 20 to < 30 ng/mL.
Four-Hour Gastric Emptying Study
Gastric emptying scintigraphy was performed after an overnight fast to assess gastric neuromuscular function [16]. Medications affecting gastrointestinal motility were stopped 48 h prior to the study. In subjects with diabetes, low blood sugar (hypoglycemia < 70 mg/dl) or high blood sugar (hyperglycemia > 270 mg/dl) was corrected or the study was rescheduled for another day. Following ingestion of a low-fat egg white sandwich meal (Egg Beaters®), imaging was performed at 0, 1, 2, and 4 h with the participant upright. Gastric retention of the meal (> 60% at 2 h and/or > 10% at 4 h) was considered delayed gastric emptying [16].
Electrogastrography and Water Load Satiety Test (WLST)
Cutaneous electrogastrography (EGG) was used to record gastric myoelectrical activity (GMA) [19,20,21]. Patients stopped proton pump inhibitors, histamine-2 receptor antagonists, prokinetics drugs, opiates, anticholinergics, cannabinoids, over-the-counter laxatives, isotonic polyethylene glycol electrolyte preparations, and prescription laxatives for 3 days before the studies. Patients fasted overnight before the test. On the morning of the study, insulin-requiring patients with diabetes injected half of their usual long-acting insulin dose. If glucose was over 270 mg/dl at the time of the test, then the glucose level was treated or the test was rescheduled. Patients were seated in a comfortable semi-reclining chair in a quiet area. EKG-type electrodes were placed in standard position on the upper abdominal surface after the skin was cleaned with alcohol wipes. Electrodes were connected to the EGG recording device (3CPM Company, Inc., Sparks Glencoe, MD) to record GMA. The EGG signal was digitized for computer analysis [19,20,21]. A 15-min baseline EGG recording was obtained. Patients then ingested water until they were “completely full” during the five-minute test period [19,20,21]. The volume of water ingested was recorded. The volume of water ingested reflects gastric capacity and gastric accommodation to the volume ingested. Ingestion of < 238 mL of water in the five-minute period is 2 SD below the mean volume ingested by healthy controls [19]. In another study, no healthy subject ingested less than 300 ml of water in the five-minute period [23]. For this study, < 300 mL of water ingested was defined as abnormal. Patients indicated the intensity of fullness, hunger, abdominal discomfort, bloating, and nausea on a 100 mm visual analog scale (VAS) before and 10, 20, and 30 min after the water was ingested [19,20,21,22]. GMA was recorded for 30 min after the water load was ingested.
The raw GMA signal was digitized and subjected to fast Fourier transformation and running spectral analysis. The power calculation in the running spectral analysis reflects the amplitude of GMA in the four frequency ranges: 1–2.5 cpm (bradygastria), 2.5–3.7 cpm (normal range), 3.7–10.0 cpm (tachygastria) and 10–15.0 cpm (duodenal/respiration range) before and after the WLST. The power in each frequency range is divided by the total power in the 1–15 cpm range. This calculation provides the percentage distribution of power for each of the four frequency ranges listed above. The percentage distribution of power in the four frequency ranges was assessed over time. The average percentage distributions of GMA were compared at baseline to each time from 1–10, 11–20, and 21–30 min after the WLST.
Statistical Analysis
We report the vitamin D levels for the entire group of patients with symptoms of gastroparesis as well as the two subgroups – delayed gastric emptying (Gp) and normal gastric emptying. Cross-sectional comparisons of three ordered vitamin D levels by characteristics at enrollment were assessed using Cochran’s X2 test for trend for binary variables and linear regression with a dose–response variable coded 0 = normal, 1 = insufficient, and 2 = deficient. Multiple ordinal logistic regression using backward stepwise selection (P > 0.05 for removal) was used to assess the direct effect of vitamin D deficiency regressed on 39 candidate variables including age, race, sex, ethnicity, summer, body mass index, HbA1c, diabetes status, gastric emptying at 1, 2 and 4 h, GCSI scores of nausea, retching, vomiting, stomach fullness, not able to finish meal, feeling excessively full, loss of appetite, bloating, stomach visibly larger, calcium, water load amount, symptoms of fullness, hunger, nausea, bloating, abdominal discomfort before and change from baseline, and percentage distributions of GMA in bradygastria, normogastria, tachygastria, and duodenal frequency at baseline and change from baseline ranges. The data analysis was generated using both SAS (SAS version 9.4, SAS Institute Inc., Cary, NC) [24] and Stata software (StataCorp. 2017. Stata Statistical Software: Release 15. College Station, TX: StataCorp LLC) [25]. All P-values are two-sided and were not corrected for multiple comparisons since these are exploratory analyses.
Results
Total Patients with Symptoms of Gastroparesis
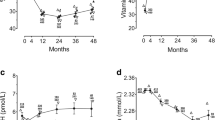
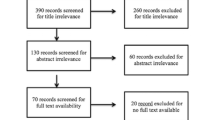
Five hundred and thirteen patients enrolled in the GpCRC Registry 2 with a WLST and Vitamin D level comprised our study cohort of patients with symptoms of Gp. Of the 513 total patients, 288 (56.1%) had low vitamin D levels with 132 being deficient and 156 being insufficient. Figure 1 shows the distribution of vitamin D levels in the entire cohort. Of these 513 symptomatic patients, 376 had delayed gastric emptying (74%) and 137 (26%) had normal gastric emptying.
Gastroparesis Patients
Of the 376 patients with Gp, 206 (54.8%) had low (deficient or insufficient) vitamin D levels. Table 1 shows the clinical characteristics, GCSI scores, gastric emptying, symptoms and GMA responses after the WLST in the patients with Gp according to normal, insufficient, and deficient vitamin D status. Patients with Gp and low vitamin D levels were significantly younger, nonwhite, and had higher BMI and HbA1c compared with patients with normal vitamin D levels (all Ps < 0.03). Patients with low vitamin D levels were less likely to be enrolled and have their vitamin D level assessed during the summer months. The prevalence of patients with disorders associated with low vitamin D levels—myocardial infarction, coronary artery disease, cerebrovascular disease, stroke were similar in the groups of patients with different vitamin D levels.
In patients with Gp and low vitamin D levels, the nausea subscore and the individual nausea, retching, vomiting scores were significantly higher compared with patients with Gp and normal vitamin D levels (all Ps < 0.004). There was no association among vitamin D levels and symptoms or subscores for fullness or bloating.
Gastric retention of the test meal at 4 h. was significantly increased (36% retention) in patients with low vitamin D compared with patients with normal vitamin D levels (31% retention, P = 0.05). There were no differences in emptying rates at 1 and 2 h.
The volume of water ingested in five minutes during the WLST by the Gp patients was 371 ± 224 mL in the normal vitamin D group and not significantly different in the insufficient and deficient vitamin D groups 401 ± 250 mL and 333 ± 178, respectively. The percentages of Gp patients who consumed less than 300 mL was 42%, 39% and 45% (P = 0.72) by vitamin D status. Nausea, bloating, and abdominal discomfort increased in intensity after the WLST but did not differ by vitamin D status. The percentage distribution of GMA in the four frequency ranges at baseline and after the WLST was not associated with vitamin D status, although normal 3 cpm GMA tended to be lower after the WLST in Gp patients with low vitamin D (P = 0.06).
Multiple regression analysis of 39 potential clinical characteristics on vitamin D levels in patients with Gp showed significant independent relationships of vitamin D levels with age, race, BMI, season, diabetes, HbA1c, nausea, percentage meal retained at 4 h, change in normal 3 cpm GMA, bradygastria and tachygastria after the WLST when adjusting for other characteristics (Table 2).
Patients with Symptoms of Gastroparesis but Normal Gastric Emptying
Of the 137 patients with symptoms of gastroparesis but normal gastric emptying, 82 patients (59.9%) had low vitamin D. The 59.9% prevalence of low vitamin D levels in patients with symptoms of gastroparesis but normal gastric emptying was not significantly different from the 54.8% prevalence of vitamin D levels in patients with gastroparesis (P = 0.32). Table 3 shows the clinical characteristics, gastric emptying, and GMA results in 137 patients with symptoms of gastroparesis but normal gastric emptying according to vitamin D status. The patients with symptoms of gastroparesis but normal gastric emptying and low vitamin D levels were significantly higher proportion of Hispanic, and had higher BMI, more diabetes and higher HbA1c levels compared with patients with normal vitamin D levels (all Ps < 0.02).
The retching and the nausea symptom severity were significantly higher in those with low vitamin D levels compared to those with normal vitamin D levels (P = 0.0002 and P = 0.009, respectively). Vitamin D levels in the symptoms of gastroparesis but normal gastric emptying patients, like those with Gp, were not associated with bloating or fullness scores.
Gastric emptying was within the normal range by definition for this patient group; differences in emptying rates within the normal range were not associated with vitamin D levels.
The average volume of water ingested during the WLST was similar for those with normal, insufficient or deficient vitamin D levels: 396 ± 243 mL vs 389 ± 230 and 392 ± 208 mL (P = 0.98), respectively. The percentages of patients with normal, insufficient, or deficient vitamin levels who consumed less than 300 mL of water in five minutes until full did not differ among the groups: 42%, 41% and 24%, respectively (P = 0.15). The intensity of symptoms after the water load were similar according to normal or decreased vitamin D status. Before ingesting the water load, the patients with deficient and insufficient vitamin D levels had significantly increased bradygastria (59% vs 61% vs 47%) compared to patients with normal vitamin D (P = 0.004) and decreased 3 cpm GMA (14% and 18%) compared with 24% 3 cpm GMA in patients with normal vitamin D (P = 0.004). The percentage tachygastria activity was similar amongst the three vitamin D groups at baseline; but after the water load was ingested, the patients with low vitamin D levels had increased tachygastria compared with baseline: mean change = + 5% and + 2% in patients with low (deficient/insufficient) vitamin D vs -2% in the patients with normal vitamin level (P = 0.01).
There were two variables that significantly (P < 0.001) differed in their relationship with vitamin D in the gastroparesis cohort and the normal gastric emptying cohort: BMI (mild relationship in Gp and strong in normal gastric emptying; interaction P = 0.001) and baseline normogastria (no relationship in Gp and strong in patients with symptoms of gastroparesis with normal gastric emptying; interaction P = 0.005) (Tables 1 and 3).
Multiple regression of vitamin D levels with 39 potential clinical characteristics in the patients with symptoms of gastroparesis but normal gastric emptying showed independent significant relationships with age, BMI, HbA1c, retching, and normal 3 cpm GMA before WLST (Table 4).
Functional Dyspepsia
Table 1 (delayed gastric emptying) and Table 3 (normal gastric emptying) list the number of patients with functional dyspepsia and its subgroups – postprandial distress syndrome and epigastric pain syndrome, according to the Rome III Diagnostic Questionnaire for Adult Functional GI Disorders for which we analyzed the gastroduodenal disorder Sect. [18]. Overall, of the total cohort of patients with symptoms of gastroparesis (both delayed and normal gastric emptying), 82.3% of patients had functional dyspepsia (81.3% of the patients with delayed gastric emptying and 85.0% of patients with normal gastric emptying). Of the total cohort, 73.4% had postprandial distress syndrome and 61.0% had epigastric pain syndrome; 52.1% had both postprandial distress syndrome and epigastric pain syndrome with 85.5% of the patients with epigastric pain syndrome also having postprandial distress syndrome. Low vitamin D levels were seen in 55.0% of patients with functional dyspepsia; with low vitamin D levels in 52.6% of the patients with postprandial distress syndrome, and 57.1% of patients with epigastric pain syndrome. These percentages of low vitamin D levels in the patients categorized as functional dyspepsia compare with the low vitamin D levels seen in 56.1% of our total cohort of patients with symptoms of gastroparesis with low vitamin D levels in 54.8% of patients with delayed gastric emptying and 59.9% of patients with symptoms of gastroparesis and normal gastric emptying.
Discussion
Our study reports that vitamin D levels were low in 54.8% of patients with gastroparesis. This prevalence of low vitamin D levels was similar in patients with symptoms of gastroparesis and normal gastric emptying (59.9%). The low vitamin D levels in these patients were associated with symptoms of nausea and vomiting and were associated with gastric neuromuscular dysfunction.
Patients with Gp are deficient in many vitamins [1, 2]. Our study shows vitamin D deficiency, as measured in the serum, is common in patients with Gp, being present in over half of patients. Our study showed the incidence of low vitamin D was similar in patients with symptoms of gastroparesis who have delayed and normal gastric emptying. Compared to U.S population prevalence in a 2022 study using NHANES data reporting 2% individuals with < 25 and 22% with 25–50 nmol/L, our patients had a much higher proportion of low vitamin D levels; however, our study patients were on average 10 years older than the NHANES population [26].
Nausea, retching, and vomiting were significantly increased in patients with symptoms of gastroparesis with low vitamin D compared with patients with normal vitamin D levels, both in patients with delayed gastric emptying and patients with normal gastric emptying. There were no relationships among vitamin D levels and fullness and bloating symptoms. The similar total GCSI scores and nausea subscale scores, the similar intensity of postprandial symptoms after the WLST and the similar incidence of low vitamin D levels in both patients with delayed as well as normal gastric emptying support the growing concept that delayed and normal gastric emptying may reflect a spectrum of related gastric neuromuscular disorders [3, 4].
How might low vitamin D and nausea and vomiting in these patients be related? First, low vitamin D was related to increased meal retained at four hours in patients with Gp. Our study supports a previous report that showed lower vitamin D levels correlated with lower rates of gastric emptying [2]. Low vitamin D may either result from severely delayed emptying or contribute to the delay. In many studies, delayed rate of gastric emptying does not correlate well or at all with symptoms associated with Gp [3, 4, 27]. A recent study indicated more severe delay in emptying, determined by high-quality scintigraphy studies, was associated with symptoms like nausea and vomiting [28]. Second, low vitamin D levels were associated with gastric dysrhythmias that can cause nausea. In Gp patients with low vitamin D levels, there was a trend towards decreased 3 cpm GMA (P = 0.06) after the WLST. Decreased 3 cpm GMA is associated with depletion of gastric ICCs, the pacemaker cells of the stomach [7, 8]. Loss of 3 cpm GMA and the presence of gastric dysrhythmias are related to nausea symptoms in a variety of conditions [13, 19, 21,22,23]. Loss of ICCs and poor GMA response to meals may contribute to nausea and delayed emptying. In addition, patients with symptoms of gastroparesis with normal gastric emptying, patients with low vitamin D had increased bradygastria activity at baseline and increased tachygastria after the WLST. Gastric ICCs are also depleted in patients with FD; but, the loss of ICCs is less compared with Gp although the patterns of dysrhythmic GMA is similar [7, 8]. Thus, nausea in patients with symptoms of Gp but normal gastric emptying may be related, in part, to low vitamin D that contributes to loss of gastric ICCs, leading to gastric myoelectric dysrhythmias, leading to symptoms of nausea and vomiting. In this study, we performed the WLST. In a prior study, we compared the nutrient drink test to WLST, finding that in patients with diabetic gastroparesis, the nutrient drink test stimulated more symptoms and changes in gastric myoelectric activity than WLST [22]. The nutrient drink test might induce more symptoms in patients with low vitamin D levels.
Low vitamin D levels may be pathophysiologically associated with Gp. For example, low vitamin D levels have been associated with the switching of M2 macrophages to pro-inflammatory M1 macrophages in ulcerative colitis and diabetic nephropathy; replenishment of vitamin D reversed these shifts [6, 29]. Depletion of ICCs is associated with infiltration of pro-inflammatory M1 macrophages in the circular smooth muscle cell layers of the corpus and antrum in patients with Gp [10, 11]. Thus, we speculate low vitamin D may contribute to immune dysregulation in the gastric muscle as well as associated changes in gastric rhythm. Based on a previous report of improved orthostatic tolerance and decreased nausea and vomiting symptoms during head up tilt table tests after replenishment [12], low vitamin D levels may also contribute to autonomic dysfunction, a potential pathophysiological feature in patients with Gp and FD.
Our study supports an association between low vitamin D levels with symptoms of nausea and vomiting and gastric myoelectrical dysfunction. The symptoms and vitamin D levels were collected at one point in time in the patients – on enrollment in the registry. According to the study protocol, the gastric emptying test could have been performed up to 6 months prior to obtaining the EGG and vitamin D levels. The association between gastric emptying rate, electrogastrography, and vitamin D levels could be influenced by the timing of these measurements. We did not explore comparing the vitamin D levels with the actual dietary intake or examine correlation between vitamin D levels and symptoms over time, which might provide more insight into cause-effect relationships between these two variables. In a prior study of ours, we found that 64% of patients with gastroparesis consumed an energy deficient diet (< 60% of daily total energy requirements). Vitamin and mineral dietary deficiencies were more prevalent in the patients consuming an energy-deficient diet, including vitamin A, thiamine, riboflavin, vitamin B6, vitamin B12, vitamin C, vitamin D, niacin, folate [28]. Vitamin D deficiency can contribute to or worsen osteoporosis; we did not collect information about the presence of osteoporosis. The effect of replenishment of vitamin D on symptoms and gastric dysfunction in patients has not yet been studied. Limitations also include the exploratory nature of the study in which multiple comparisons were made.
In conclusion, vitamin D levels were low in 56% of patients with symptoms of gastroparesis, being similar in those with delayed gastric emptying and normal gastric emptying. Low vitamin D was associated with the symptoms of nausea, retching, and vomiting. Low vitamin D may be related to nausea through gastric neuromuscular dysfunction, potential relationships that need further investigation. This study lays the framework for the next level of investigation, replenishment of vitamin D in patients with symptoms of gastroparesis who have low vitamin D levels and see if this improves their gastric neuromuscular dysfunction and symptoms of gastroparesis. Until this study is performed, we advocate assessing vitamin D levels in patients with symptoms of gastroparesis and treatment with exogenous vitamin D if the patient is deficient in vitamin D.
Data availability
The data that support the findings of this study are openly available in NIDDK data repository at https://repository.niddk.nih.gov/home/.
References
Parkman HP, Yates KP, Hasler WL, Nguyan L, Pasricha PJ, Snape WJ, Farrugia G, Calles J, Koch KL et al. Dietary intake and nutritional deficiencies in patients with diabetic or idiopathic gastroparesis. Gastroenterology 2011;141:486–498.
Kedar A, Nikitina Y, Henry OR et al. Gastric dysmotility and low serum vitamin D levels in patients with gastroparesis. Horm Metab Res 2013;45:47–53.
Pasricha PJ, Colvin R, Yakes K, Hasler WL, Abell TL, Unalp-Arida A, Nguyen L, Farrugia G, Koch KL, Parkman HP, Snape WJ, Lee L, Tonascia J, Hamilton FA. Clinical characteristics of patients with chronic unexplained nausea and vomiting and normal gastric emptying. Clin Gastroenterol Hepatol 2011;7:567–576.
Pasricha PJ, Grover M, Yates KP, Abell TL, Bernard CE, Koch KL, McCallum RW, Sarosiek I, Kuo B, Bulat R, Chen J, Shulman RJ, Lee L, Tonascia J, Miriel LA, Hamilton F, Farrugia G, Parkman HP. Functional dyspepsia and gastroparesis in tertiary care are interchangeable syndromes with common clinical and pathological features. Gastroenterology 2021;160:2006–2017.
Dankers W, Colin EM, van Hamburg JP, Lubberts E. Vitamin D in autoimmunity: Molecular mechanisms and therapeutic potential. Front Immunol 2017;7:1–26.
Ubatan J, Mitsuhashi S, Zenlea T et al. Low serum vitamin D during remission increases risk of clinical relapse in patients with ulcerative colitis. Clin Gastroenterol Hepatol 2017;15:240–246.
O'Grady G, Angeli TR, Du P, et al. Abnormal initiation and conduction of slow-wave activity in gastroparesis, defined by high-resolution electrical mapping. Gastroenterology 2012;143:589–98.e1–3.
Angeli TR, Cheng LK, Du P et al. Loss of interstitial cells of Cajal and patterns of gastric dysrhythmia in patients with chronic unexplained nausea and vomiting. Gastroenterology 2015;149:56-66.e5.
Farrugia G, Lurken MS, Bernard CE, Faussone-Pellegrini MS, Smyrk TC, Parkman HP, Abell TL, Snape WJ, Hasler WL, Unalp-Arida A, Nguyen L, Koch KL. Cellular changes in diabetic and idiopathic gastroparesis. Gastroenterol 2011;140:1575–1585.
Grover M, Bernard CE, Pasricha PJ, et al. Diabetic and idiopathic gastroparesis is associated with loss of CD206-positivie macrophages in the gastric antrum. Neurogastroenterol Motil 2017;29:https://doi.org/10.1111/nmo.13018.
Bernard CE, Gibbons SJ, Mann IS et al. Association of low numbers of CD206-positive cells with loss of ICC in the gastric body of patients with diabetic gastroparesis. Neurogastroenterol Motil 2014;26:1275–1284.
Shaltout HA, Glock MS. Low serum vitamin D is associated with greater impairment in autonomic function upon head up tilt in children with orthostatic intolerance. Hypertension 2015;66:AP633.
Stefi E, Shaltout H, Brown A, Koch K. Gastric myoelectrical activity and autonomic nervous system abnormalities in patients with chronic unexplained nausea and vomiting and in patients with gastroparesis. Int Gastroenterol Hepatal 2019;163–171.
Nguyen L, Wilson LA, Miriel L, Pasricha PJ, Kuo B, Hasler WL, McCallum RW, Sarosiek I, Koch KL, Snape WJ, Farrugia G, Grover M, Clarke J, Parkman HP, Tonascia J, Hamilton F, Abell TL; NIDDK Gastroparesis Clinical Research Consortium (GpCRC). Autonomic function in gastroparesis and chronic unexplained nausea and vomiting: Relationship with etiology, gastric emptying, and symptom severity. Neurogastroenterol Motil 2020;32:e13810.
Shaltout HA. Vitamin D supplementation improves cardiovascular outcome and response to head up tilt in adolescents suffering from syncope. Hypertension 2018;70:P396.
Tougas G, Eaker EY, Abell TL, Abrahamsson H, Boivin M, Chen J, Hocking MP, Quigley EMM, Koch KL, Tokayer AZ, Stanghellini V, Chen Y, Huizinga JD, Ryden J, Bourgeois I, McCallum RW. Assessment of gastric emptying using a low fat meal: Establishment of international control values. Am J Gastroenterol 2000;95:1456–1462.
Revicki DA, Rentz AM, Dubois D, Kahrilas P, Stanghellini V, Talley NJ, Tack J. Development and validation of a patient-assessed gastroparesis symptom severity measure: the Gastroparesis Cardinal Symptom Index. Aliment Pharmacol Ther 2003;18:141–150.
Drossman DA. The functional gastrointestinal disorders and the Rome III process. Gastroenterology 2006;130:1377–1390.
Koch KL, Hong S-P, Xu L. Reproducibility of gastric myoelectrical activity and the water load test in patients with dysmotility-like dyspepsia symptoms and in control subjects. J Clin Gastroenterol 2000;31:125–129.
Koch KL. Electrogastrography for Suspected Gastroparesis. In Gastroparesis: Pathophysiology, Clinical Presentation, Diagnosis and Treatment. Eds. R. McCallum, H Parkman. Elsevier, 2020.
Koch KL, van Natta M, Parkman HP, Grover M, Abell TL, McCallum RW, Shaltout HA, Sarosiek I, Farrugia G, Shulman RJ, Tonascia J, Miriel L, Hamilton F, Pasricha PJ. Effect of liquid and solid test meals on symptoms and gastric myoelectrical activity in patients with gastroparesis and functional dyspepsia. Neurogastroenterol Motil 2023;35:e14376.
Koch KL, Hasler WL, Van Natta M, Calles-Escandon J, Grover M, Pasricha PJ, Snape WJ, Parkman HP, Abell TL, McCallum RW, Nguyen LA, Sarosiek I, Farrugia G, Tonascia J, Lee L, Miriel L, Hamilton F; NIDDK Gastroparesis Clinical Research Consortium. Satiety testing in diabetic gastroparesis: Effects of insulin pump therapy with continuous glucose monitoring on upper gastrointestinal symptoms and gastric myoelectrical activity. Neurogastroenterol Motil 2020;32:e13720.
Jones MP, Hoffman S, Shah D et al. The water load test: Observations from healthy controls and patients with functional dyspepsia. Am J Physiol 2003;284:G896-904.
SAS version 9.4, SAS Institute Inc., Cary, NC
StataCorp. 2017. Stata Statistical Software: Release 15. College Station, TX: StataCorp LLC)
Cui A, Xiao P, Ma Y, Fan Z, Zhou F, Zheng J, Zhang L. Prevalence, trend, and predictor analyses of vitamin D deficiency in the US population, 2001–2018. Front Nutr 2022;9:965376.
Janssen P, Harris S, Jones M et al. The relation between symptoms improvement and gastric emptying in the treatment of diabetic and idiopathic gastroparesis. Am J Gastroenterol 2013;108:1382–1391.
Vijayvargiya P, Jameie-Oskooei S, Camilleri M et al. Association between delayed gastric emptying and upper gastrointestinal symptoms: a systematic review and meta-analysis. Gut 2019;68:804–813.
Zhang X, Zhao Y, Zhu X et al. Active vitamin D regulates macrophage M1/M2 phenotypes via the Stat-1-TREM-1 pathway in diabetic nephrophathy. J Cell Physiol 2019;234:6917–6926.
Parkman HP, Yates KP, Hasler WL, Nguyen L, Pasricha PJ, Snape WJ, Farrugia G, Calles J, Koch KL, Abell TL, McCallum RW, Lee L, Unalp-Arida A, Tonascia J, Hamilton F for the NIDDK Gastroparesis Clinical Research Consortium (GpCRC). Dietary intake and nutritional deficiencies in patients with diabetic or idiopathic gastroparesis. Gastroenterology 2011; 141:486–498.
Acknowledgments
Jorge Calles-Escandon, MD (Endocrinology Section, MetroHealth Medical Center, Cleveland, OH); Hossam A. Shaltout, PhD (Cardiovascular Sciences Center, Wake Forest University) Winston-Salem, NC); William L. Hasler, MD (Department of Gastroenterology, Mayo Clinic; Scottsdale, AZ); William J. Snape, MD (California Pacific Medical Center, San Francisco, CA); Linda A. Nguyen, MD (Division of Gastroenterology, Stanford University, Palo Alto, CA); and Frank Hamilton (National Institutes of Health – NIDDK, Bethesda, MD) for help in conceptualization and performance of this study
Funding
The Gastroparesis Consortium (GpCRC) is supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (grants U01DK112193, U01DK112194, U01DK073983, U01DK073975, U01DK074035, U01DK074007, U01DK073974, U24DK074008) and the National Center for Advancing Translational Sciences (NCATS) (grants UL1TR000424, UL1TR000135). The authors thank the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) for its support of the GpCRC and this research. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author information
Authors and Affiliations
Contributions
Kenneth L. Koch, MD: study design, enrollment of patients; study supervision; writing manuscript. Henry P. Parkman, MD: study design, enrollment of patients, study concept and design; analysis and interpretation of data; writing manuscript. Katharine P. Yates, PhD: analysis and interpretation of data; critical revision of the manuscript for important intellectual content. Mark L. Van Natta, MHS: analysis and interpretation of data; critical revision of the manuscript for important intellectual content. Madhusudan Grover, MBBS: critical revision of the manuscript for important intellectual content. Gianrico Farrugia, MD: critical revision of the manuscript for important intellectual content. Thomas L. Abell, MD: enrollment of patients; critical revision of the manuscript for important intellectual content. Richard W. McCallum, MD: study concept and design; enrollment of patients; critical revision of manuscript for important intellectual content. Irene Sarosiek, MD: enrollment of patients; critical revision of the manuscript for important intellectual content. Braden Kuo, MD: study concept and design; critical revision of manuscript for important intellectual content. Robert J. Shulman, MD: critical revision of manuscript for important intellectual content. Laura Miriel, BS: study design; reading of manuscript for important intellectual control; study supervision. James Tonascia, PhD: study design; analysis and interpretation of data; critical revision of manuscript for important intellectual content. Pankaj J. Pasricha, MD: study design; enrollment of patients; critical revision of manuscript for important intellectual content.
Corresponding author
Ethics declarations
Conflict of interest
Kenneth Koch is a shareholder in the 3CPM Company.
ClinicalTrials.gov Identifier
NCT01696747.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Koch, K.L., Parkman, H.P., Yates, K.P. et al. Low Vitamin D Levels in Patients with Symptoms of Gastroparesis: Relationships with Nausea and Vomiting, Gastric Emptying and Gastric Myoelectrical Activity. Dig Dis Sci (2024). https://doi.org/10.1007/s10620-024-08520-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10620-024-08520-8