Abstract
Background
Methotrexate (MTX) combination therapy with biological agents has gained increasing interest. Here, we assessed the efficacy and tolerability of the MTX combination therapy in patients with Crohn’s disease (CD).
Methods
We performed a multicenter observational study with 185 patients with CD with MTX and biologics combination therapy; the patients were recruited from three IBD Clinics in Korea. We evaluated the outcomes of the MTX combination therapy and examined the predictive factors of clinical and endoscopic remission.
Results
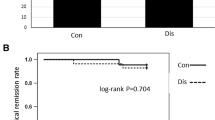
MTX was administered orally to 62.7% of patients; the mean dose was 15.5 mg per week, and the mean treatment duration was 36 months. Of the 169 patients treated with MTX combination therapy for over 6 months, the steroid-free clinical remission rates were 34.3%, 26.0%, 29.8%, and 32.7% at 4, 12, 18, and 24 months, respectively. Previous thiopurine use was a significant negatively associated independent factor (p < 0.001), and a higher dose of MTX (≥ 15 mg/week) was a positively associated independent factor of steroid-free clinical remission (p = 0.035). Ninety-six patients underwent follow-up endoscopy after 28 months, and 36 (37.5%) achieved endoscopic remission. Longer disease duration (p = 0.006), ileocolonic type of Montreal location (p = 0.036), and baseline C-reactive protein (CRP) level of more than 5 mg/L (p = 0.035) were significant negatively associated independent factors and a higher dose of MTX (≥ 15 mg/week) was a positively associated independent factor of endoscopic remission (p = 0.037).
Conclusions
MTX combination therapy with biologics was effective and tolerable in patients with CD.


Similar content being viewed by others
Abbreviations
- CD:
-
Crohn’s disease
- TNF:
-
Tumor necrosis factor
- MTX:
-
Methotrexate
- 6-MP:
-
6-Mercaptopurine
- FDA:
-
Food and Drug Administration
- ATI:
-
Antibodies to infliximab
- CBC:
-
Complete blood count
- CDAI:
-
Crohn’s Disease Activity Index
- CRP:
-
C-reactive protein
- SES-CD:
-
Simple Endoscopic Score for Crohn’s Disease
- BMI:
-
Body mass index
- OR:
-
Odds ratio
- CI:
-
Confidence interval
- HR:
-
Hazard ratio
References
Torres J, Bonovas S, Doherty G et al. ECCO guidelines on therapeutics in Crohn’s disease: medical treatment. J Crohns Colitis. 2020;14:4–22.
Otake H, Matsumoto S, Mashima H. Long-term clinical and real-world experience with Crohn’s disease treated with anti-tumor necrosis factor-alpha antibodies. Intest Res. 2022;20:464–474.
Maser EA, Villela R, Silverberg MS, Greenberg GR. Association of trough serum infliximab to clinical outcome after scheduled maintenance treatment for Crohn’s disease. Clin Gastroenterol Hepatol. 2006;4:1248–1254.
Kennedy NA, Heap GA, Green HD et al. Predictors of anti-TNF treatment failure in anti-TNF-naive patients with active luminal Crohn’s disease: a prospective, multicentre, cohort study. Lancet Gastroenterol Hepatol. 2019;4:341–353.
Rosh JR, Gross T, Mamula P, Griffiths A, Hyams J. Hepatosplenic T-cell lymphoma in adolescents and young adults with Crohn’s disease: a cautionary tale? Inflamm Bowel Dis. 2007;13:1024–1030.
Mackey AC, Green L, Leptak C, Avigan M. Hepatosplenic T cell lymphoma associated with infliximab use in young patients treated for inflammatory bowel disease: update. J Pediatr Gastroenterol Nutr. 2009;48:386–388.
Ansari A, Arenas M, Greenfield SM et al. Prospective evaluation of the pharmacogenetics of azathioprine in the treatment of inflammatory bowel disease. Aliment Pharmacol Ther. 2008;28:973–983.
Kim JH, Cheon JH, Kim WH. The frequency and the course of the adverse effects of azathioprine/6-mercaptopurine treatment in patients with inflammatory bowel disease. Korean J Gastroenterol. 2008;51:291–297.
Herfarth HH, Kappelman MD, Long MD, Isaacs KL. Use of methotrexate in the treatment of inflammatory bowel diseases. Inflamm Bowel Dis. 2016;22:224–233.
Gabbani T, Deiana S, Lunardi S, Manetti N, Annese V. Safety profile of methotrexate in inflammatory bowel disease. Expert Opin Drug Saf. 2016;15:1427–1437.
Feagan BG, McDonald JW, Panaccione R et al. Methotrexate in combination with infliximab is no more effective than infliximab alone in patients with Crohn’s disease. Gastroenterology. 2014;146:681–688.
Vermeire S, Noman M, Van Assche G et al. Effectiveness of concomitant immunosuppressive therapy in suppressing the formation of antibodies to infliximab in Crohn’s disease. Gut. 2007;56:1226–1231.
Carbonnel F, Colombel JF, Filippi J et al. Methotrexate is not superior to placebo for inducing steroid-free remission, but induces steroid-free clinical remission in a larger proportion of patients with ulcerative colitis. Gastroenterology. 2016;150:380–388.
Daperno M, D’Haens G, Van Assche G et al. Development and validation of a new, simplified endoscopic activity score for Crohn’s disease: the SES-CD. Gastrointest Endosc. 2004;60:505–512.
Chande N, Wang Y, MacDonald JK, McDonald JW. Methotrexate for induction of remission in ulcerative colitis. Cochrane Database Syst Rev. 2014;2014:CD006618.
Koh SJ, Hong SN, Park SK et al. Korean clinical practice guidelines on biologics for moderate to severe Crohn’s disease. Intest Res. 2022.
Hawthorne AB. Methotrexate: a useful alternative in Crohn’s disease? Gut. 2001;49:9–10.
Rampton DS. Methotrexate in Crohn’s disease. Gut. 2001;48:790–791.
Rosh JR. The current role of methotrexate in patients with inflammatory bowel disease. Gastroenterol Hepatol. 2020;16:43–46.
Narula N, Peyrin-Biroulet L, Colombel JF. Combination therapy with methotrexate in inflammatory bowel disease: time to COMMIT? Gastroenterology. 2014;146:608–611.
Colman RJ, Rubin DT. Optimal doses of methotrexate combined with anti-TNF therapy to maintain clinical remission in inflammatory bowel disease. J Crohns Colitis. 2015;9:312–317.
Borren NZ, Luther J, Colizzo FP et al. Low-dose methotrexate has similar outcomes to high-dose methotrexate in combination with anti-TNF therapy in inflammatory bowel diseases. J Crohns Colitis. 2019;13:990–995.
Henriksen M, Jahnsen J, Lygren I et al. C-reactive protein: a predictive factor and marker of inflammation in inflammatory bowel disease. Results from a prospective population-based study. Gut. 2008;57:1518–1523.
Colombel JF, Sandborn WJ, Reinisch W et al. Infliximab, azathioprine, or combination therapy for Crohn’s disease. N Engl J Med. 2010;362:1383–1395.
Armuzzi A, Pugliese D, Danese S et al. Long-term combination therapy with infliximab plus azathioprine predicts sustained steroid-free clinical benefit in steroid-dependent ulcerative colitis. Inflamm Bowel Dis. 2014;20:1368–1374.
Maeda T, Sakuraba H, Hiraga H et al. Long-term efficacy and tolerability of dose-adjusted thiopurine treatment in maintaining remission in inflammatory bowel disease patients with NUDT15 heterozygosity. Intest Res. 2022;20:90–100.
Kim YZ, Kang B, Kim ES, et al. Efficacy of combined initial treatment of methotrexate with infliximab in pediatric Crohn’s disease: a pilot study. Biomedicines. 2023;11.
Shea B, Swinden MV, Tanjong Ghogomu E et al. Folic acid and folinic acid for reducing side effects in patients receiving methotrexate for rheumatoid arthritis. Cochrane Database Syst Rev. 2013;2013:CD000951.
Reich K, Langley RG, Papp KA et al. A 52-week trial comparing briakinumab with methotrexate in patients with psoriasis. N Engl J Med. 2011;365:1586–1596.
Fournier MR, Klein J, Minuk GY, Bernstein CN. Changes in liver biochemistry during methotrexate use for inflammatory bowel disease. Am J Gastroenterol. 2010;105:1620–1626.
Saibeni S, Bollani S, Losco A et al. The use of methotrexate for treatment of inflammatory bowel disease in clinical practice. Dig Liver Dis. 2012;44:123–127.
Wahed M, Louis-Auguste JR, Baxter LM et al. Efficacy of methotrexate in Crohn’s disease and ulcerative colitis patients unresponsive or intolerant to azathioprine/mercaptopurine. Aliment Pharmacol Ther. 2009;30:614–620.
Seinen ML, Ponsioen CY, de Boer NK et al. Sustained clinical benefit and tolerability of methotrexate monotherapy after thiopurine therapy in patients with Crohn’s disease. Clin Gastroenterol Hepatol. 2013;11:667–672.
Park JJ, Yang SK, Ye BD et al. Second Korean guidelines for the management of Crohn’s disease. Intest Res. 2017;15:38–67.
Carter MJ, Lobo AJ, Travis SP, Ibd Section BSoG. Guidelines for the management of inflammatory bowel disease in adults. Gut. 2004;53:V1–V16.
Dignass A, Eliakim R, Magro F et al. Second European evidence-based consensus on the diagnosis and management of ulcerative colitis part 1: definitions and diagnosis. J Crohns Colitis. 2012;6:965–990.
Acknowledgments
None.
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declare that they have no competing interest.
Ethical considerations
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Park, J., Chun, J., Park, S.J. et al. Effectiveness and Tolerability of Methotrexate Combined with Biologics in Patients with Crohn’s Disease: A Multicenter Observational Study. Dig Dis Sci 69, 901–910 (2024). https://doi.org/10.1007/s10620-023-08237-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-023-08237-0