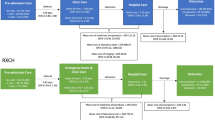
Abstract
Background
Liver transplant recipients (LTR) and patients with chronic liver disease (CLD) are at an increased risk of infections.
Aims
The objective of our study was to assess the incidence, and impact of vaccine preventable illness (VPI) on outcomes in LTR.
Methods
National Inpatient Sample (NIS) 2016–2020 was used to identify adults (age > 18) hospitalized LTR using ICD-10 codes. Data were collected on patient demographics, hospital characteristics, etiology of liver disease, hepatic decompensations and outcomes. Patients were stratified into two groups based on the presence or absence of VPI. Multivariate logistic regression analysis was performed to identify the association between VPI and outcomes.
Results
Out of 170,650 hospitalized LTR, 13.5% of the patients had VPI. The most common VPI was noted to be influenza (10.7%), followed by pneumococcal infection (2.7%). Incidence of mortality (6.9% vs. 1.6%, p < 0.001), ICU admissions (14.3% vs. 3.4%, p < 0.001), and acute kidney injury (AKI) (43.7% vs 37.35%, p < 0.001) was higher in the VPI group.
Conclusion
More than 13% of the LT hospitalizations had concomitant VPI. VPI in LTR was associated with worse outcomes. Our data suggests the need to identify factors associated with reduced vaccination rates and identify strategies to improve vaccination rates and responses in these patients.
Graphical Abstract



Similar content being viewed by others
References
Tuchendler E, Tuchendler PK, Madej G. Immunodeficiency caused by cirrhosis. Clin Exp Hepatol 2018;4:158–164.
Gustot T, Durand F, Lebrec D, Vincent JL, Moreau R. Severe sepsis in cirrhosis. Hepatology (Baltimore, Md.) 2009;50:2022–2033.
Gustot T, Fernandez J, Garcia E et al. Clinical course of acute-on-chronic liver failure syndrome and effects on prognosis. Hepatology 2015;2:243–252. https://doi.org/10.1002/hep.27849.
Jalan-Sakrikar N, Brevini T, Huebert RC, Sampaziotis F. Organoids and regenerative hepatology. Hepatology (Baltimore, Md) 2023;77:305.
Cindy Young. (2022, January 11). All-time records again set in 2021 for organ transplants, organ donation from deceased donors. UNOS. Retrieved April 29, 2023, from https://unos.org/news/2021-all-time-records-organ-transplants-deceased-donor-donation/
Pilar Hernandez MD, Martin P, Simkins J. Infectious complications after liver transplantation. Gastroenterol Hepatol 2015;11:741–753.
Romero FA, Razonable RR. Infections in liver transplant recipients. World J Hepatol 2011;3:83–92.
Rolak S, Said A, German R, Hayney MS, Caldera F. Optimizing immunization strategies in adult patients with chronic liver disease and liver transplant recipients. Gastroenterol Hepatol 2022;18:196–206.
Caballero-Marcos A, Salcedo M, Alonso-Fernández R, Rodríguez-Perálvarez M, Spanish Society of Liver Transplantation (SETH) et al. Changes in humoral immune response after SARS-CoV-2 infection in liver transplant recipients compared to immunocompetent patients. Am J Transplant 2021;21:2876–2884. https://doi.org/10.1111/ajt.16599.
Rubin LG, Levin MJ, Ljungman P, Davies EG, Infectious Diseases Society of America, et al. 2013 IDSA clinical practice guideline for vaccination of the immunocompromised host. Clin Infect Dis 2014;58:44–100.
Danziger-Isakov L, Kumar D. Vaccination of solid organ transplant candidates and recipients: guidelines from the American society of transplantation infectious diseases community of practice. Clin Transplant. 2019;33:e13563.
Feldman AG, Atkinson K, Wilson K, Kumar D. Under-immunization of the solid organ transplant population: an urgent problem with potential digital health solutions. Am J Transplant 2020;20:34–39.
Kim YJ, Kim SI. Vaccination strategies in patients with solid organ transplant: Evidences and future perspectives. Clin Exp Vaccine Res 2016;5:125–131.
Laue T, Demir Z, Debray D, Cananzi M et al. Under-vaccination in pediatric liver transplant candidates with acute and chronic liver disease-a retrospective observational study of the European Reference Network TransplantChild. Children (Basel, Switzerland) 2021;8:675. https://doi.org/10.3390/children8080675.
Danziger-Isakov L, Kumar D, AST Infectious Diseases Community of Practice. Vaccination in solid organ transplantation. Am J Transplant 2013;13:311–317. https://doi.org/10.1111/ajt.12122.
Invasive Pneumococcal Disease in Solid Organ Transplant Recipients—10-Year Prospective Population SurveillanceKumar, D. et al.American Journal of Transplantation, Volume 7, Issue 5, 1209 - 1214
HCUP National Inpatient Sample (NIS). Healthcare cost and utilization project (HCUP). 2016–2019. Agency for healthcare research and quality. Rockville, MD.
Elixhauser Comorbidity Software Refined for ICD-10-CM Healthcare Cost and Utilization Project (HCUP) . October 2021. Agency for healthcare research and quality. Rockville, MD.
Ison MG. Influenza prevention and treatment in transplant recipients and immunocompromised hosts. Influenza Other Respir Viruses 2013;7:60–66. https://doi.org/10.1111/irv.12170.
Duchini A, Hendry RM, Redfield DC, Pockros PJ. Influenza infection in patients before and after liver transplantation. Liver Transpl. 2000;6:531–542. https://doi.org/10.1053/jlts.2000.9738.
Kumar D, Ferreira VH, Blumberg E, Silveira F et al. A 5-year prospective multicenter evaluation of influenza infection in transplant recipients. Clin Infect Dis 2018;67:1322–1329. https://doi.org/10.1093/cid/ciy294.
Weltermann B, Herwig A, Dehnen D, Herzer K. Vaccination status of pneumococcal and other vaccines in 444 liver transplant patients compared to a representative population sample. Ann Transplant. 2016;21:200–207. https://doi.org/10.12659/aot.896436.
Kumar D, Chen MH, Wong G et al. A randomized, double-blind, placebo-controlled trial to evaluate the prime-boost strategy for pneumococcal vaccination in adult liver transplant recipients. Clin Infect Dis. 2008;47:885–892. https://doi.org/10.1086/591537.
McCashland TM, Preheim LC, Gentry MJ. Pneumococcal vaccine response in cirrhosis and liver transplantation. J Infect Dis. 2000;181:757–760. https://doi.org/10.1086/315245.
Kumar D, Humar A, Plevneshi A, Green K, Prasad GV, Siegal D, Toronto Invasive Bacterial Diseases Network, McGeer A. Invasive pneumococcal disease in solid organ transplant recipients–10-year prospective population surveillance. Am J Transplant 2007;7:1209–1214.
Rezahosseini O, Møller DL, Sørensen SS, Perch M et al. An observational prospective cohort study of incidence and outcome of streptococcus pneumoniae and Hemophilus influenzae infections in adult solid organ transplant recipients. Microorganisms 2021;9:1371. https://doi.org/10.3390/microorganisms9071371.
Levitsky J, Kalil A, Meza JL, Hurst GE, Freifeld A. Herpes zoster infection after liver transplantation: a case-control study. Liver Transpl 2005;11:320–325.
Kim W, Kim S, Oh J, Jeong YJ et al. Incidence and risk factors for herpes zoster after adult liver transplantation. Ann Surg Treat Res. 2019;96:95–99. https://doi.org/10.4174/astr.2019.96.2.95.
Pergam SA, Forsberg CW, Boeckh MJ et al. Herpes zoster incidence in a multicenter cohort of solid organ transplant recipients. Transpl Infect Dis. 2011;13:15–23.
Hirzel C, L’Huillier AG, Ferreira VH, Marinelli T et al. Safety and immunogenicity of adjuvanted recombinant subunit herpes zoster vaccine in lung transplant recipients. Am J Transpl 2021;21:2246–2253. https://doi.org/10.1111/ajt.16534.
Vink P, Ramon Torrell JM, Sanchez Fructuoso A, Kim SJ, Z-041 Study Group et al. Immunogenicity and safety of the adjuvanted recombinant zoster vaccine in chronically immunosuppressed adults following renal transplant: a phase 3, Randomized Clinical Trial. Clin Infect Dis 2020;70:181–190. https://doi.org/10.1093/cid/ciz177.
Anderson TC, Masters NB, Guo A, Shepersky L et al. Use of recombinant zoster vaccine in immunocompromised adults aged ≥19 years: recommendations of the Advisory Committee on Immunization Practices—United States. MMWR 2022;71:80–84. https://doi.org/10.15585/mmwr.mm7103a2.
Wang L, Verschuuren EA, van Leer-Buter CC, Bakker SJ, De Joode AA, Westra J, Bos NA. Herpes zoster and immunogenicity and safety of zoster vaccines in transplant patients: a narrative review of the literature. Front Immunol 2018;9:1632. https://doi.org/10.3389/fimmu.2018.01632.
Dooling KL, Guo A, Patel M et al. Recommendations of the Advisory Committee on Immunization Practices for use of herpes zoster vaccines. MMWR Morb Mortal Wkly Rep 2018;67:103–8. https://doi.org/10.15585/mmwr.mm6703a5.
Harpaz R, Ortega-Sanchez IR, Seward JF, Advisory Committee on Immunization Practices; CDC. Prevention of herpes zoster: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2008;57(No. RR-5):1–30.
Walti LN, Mugglin C, Mombelli M, Manuel O, Swiss Transplant Cohort Study et al. Vaccine-preventable infections among solid organ transplant recipients in Switzerland. JAMA Netw Open 2023;6:687.
Arentoft NS, Møller DL, Knudsen AD, Abdulovski R et al. Influenza in liver and kidney transplant recipients: incidence and outcomes. Microbiol Spectr 2023;11:e0322622.
Stockwell MS, Fiks AG. Utilizing health information technology to improve vaccine communication and coverage. Hum Vaccines Immunother 2013;9:1802–1811.
Wilson K, Atkinson KM, Westeinde J. Apps for immunization: Leveraging mobile devices to place the individual at the center of care. Hum Vaccin Immunother 2015;11:2395–2399.
Szilagyi PG, Bordley C, Vann JC, Chelminski A, Kraus RM, Margolis PA et al. Effect of patient reminder/recall interventions on immunization rates: a review. Jama 2000;284:1820–1827.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sohal, A., Kohli, I., Chaudhry, H. et al. Vaccine-Preventable Illness Leads to Adverse Outcomes in Liver Transplant Recipients. Dig Dis Sci 69, 588–595 (2024). https://doi.org/10.1007/s10620-023-08202-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-023-08202-x